Mild Sustainable Amide Alkylation Protocol Enables a Broad Orthogonal Scope
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 1
Abstract
Herein, the development of a mild sustainable protocol to couple primary alkyl chlorides and bromides with amides is described. In contrast to current methodologies, our system does not require the use of strongly basic conditions, high temperatures, or the addition of an organometallic catalyst, thereby enabling access to a remarkably orthogonal scope. K3PO4 is used to facilitate the formation of secondary and tertiary amides, which are ubiquitous scaffolds in bioactive molecules and natural products. Alkylated amide products are obtained in good to excellent yields, with no substantial limitations observed based on the steric and electronic properties of either coupling partner.
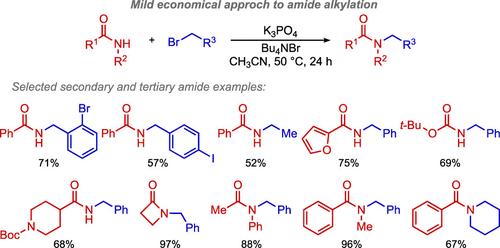
温和可持续的酰胺烷基化协议使广泛的正交范围
本文描述了一种温和的可持续方案,将伯烷基氯化物和溴化物与酰胺偶联。与目前的方法相比,我们的系统不需要使用强碱条件、高温或添加有机金属催化剂,因此可以达到显著的正交范围。K3PO4用于促进二级和三级酰胺的形成,这些酰胺是生物活性分子和天然产物中普遍存在的支架。烷基化酰胺产品的产率很高,没有观察到基于任何偶联伙伴的空间和电子性质的实质性限制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


