Combination of peroxymonosulfate and Fe(Ⅵ) for enhanced degradation of sulfamethoxazole: The overlooked roles of high-valent iron species
Abstract
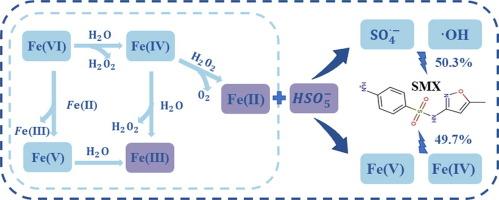
Sulphate radicals (SO4−) has been widely considered as the predominant active species for pollutants degradation by the combination of ferrate (Fe(VI)) and peroxymonosulfate (PMS). However, this study for the first time revealed the important roles of high-valent iron species in the degradation of sulfamethoxazole (SMX) by Fe(VI)/PMS system. The competitive oxidation kinetics results indicated that when the molar ratio of PMS/Fe(VI) was 1:1 at neutral pH, the contributions of high-valent iron species and free radicals to SMX degradation were 49.3% and 50.7%, respectively. By comparing the Fe(VI)/PMS and Fe(VI) systems, it was found that PMS could promote the production of high-valent iron species. Density functional theory calculations showed that SMX was more susceptible to electrophilic attack initiated by high-valent iron species rather than free radicals. Because of their different oxidative reactivities toward different organic contaminants, the high-valent iron species and free radicals contributed differently to abating different organic contaminants. Significantly, using methyl phenyl sulfoxide (PMSO) as a probe of high-valent iron species indicated that the dominant ROS changed from high-valent iron species to free radicals with an increase in the PMS/Fe(Ⅵ) molar ratio at pH 7.0. The results of this work may facilitate the process regulation and application of Fe(VI)/PMS systems in water/wastewater treatment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


