Blending fiber-shaped long-range conductive additives for better battery performance: Mechanism study based on heterogeneous electrode model
Abstract
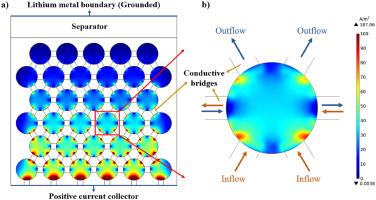
Conductive additives in lithium-ion batteries are indispensable for constructing electronic conductive networks and enabling continuous electrochemical reactions. Fine carbon black powder, typically composed of small round particles with a diameter of tens of nanometers, is the most commonly used conductive additive. The experimental results in some literature indicate that blending fiber-shaped conductive additives can help boost battery performance. The heterogenous electrode model is employed in this study to explore the distribution of electronic current density within lithium-ions batteries, demonstrate the benefits of blending fiber-shaped long-range conductive additives, and shed light on its mechanism. Our findings show that the introduction of long-range conductive additives leads to reallocation of the electron flow: as the proportion/weight ratio of long-range conductive additives increases, the electronic current undertaken by all conductive additives increases, while that undertaken by the active particles decreases. Without changing the total wt.% of conductive additives in batteries, the ohmic resistance of the electrode and the whole cell can be significantly reduced due to more electrons passing through compositions with better conductivity. These results help researchers gain a better understanding on the correlation between conductive network geometry and electron transfer effectiveness.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


