Ultrasound molecular imaging of p32 protein translocation for evaluation of tumor metastasis
Abstract
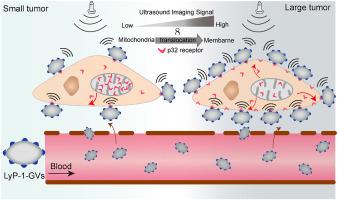
Protein translocation is an essential process for living cells to respond to different physiological, pathological or environmental stimuli. However, its abnormal occurrence usually results in undesirable outcomes such as tumors. To date, there is still a lack of appropriate methods to detect this event in live animals in a real-time manner. Here, we identified the gradually increased cell-surface translocation of p32 protein from mitochondria during tumor progression. LyP-1-modified gas vesicles (LyP-1-GVs) were developed through conjugating LyP-1 (p32-targeting peptide) to the biosynthetic GVs to monitor the cell-surface level of p32 translocation. The resulting LyP-1-GVs have about 200 nm particle size and good tumor cell targeting performance. Upon systemic administration, LyP-1-GVs can traverse through blood vessels and bind to the tumor cells, producing strong contrast imaging signals in comparison with the non-targeted GVs. The contrast imaging signals correlate well with the cell-surface translocation level of p32 protein and tumor metastatic ability. To our knowledge, this is the first report about the in vivo detection of protein translocation to cell membrane from mitochondria by ultrasound molecular imaging. Our study provides a new strategy to explore the molecular events of protein membrane translocations for evaluation of tumor metastasis at the live animal level.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


