Preparation of lauryl thioglycoside of N-glycolylneuraminic acid (Neu5Gc) as a useful glycosyl donor for assembly of an oligosaccharide containing Neu5Gc
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Efficient synthesis of lauryl thioglycoside of Neu5Gc was accomplished from a known thioglycoside of Neu5Ac. The reactivities of the Neu5Gc thioglycoside for O-glycosidation by means of TMSOTf-NIS as a promoter system were investigated, and the thioglycoside showed good activities with simple alcohols. In addition to the simple alcohols, the reactivities of lauryl thioglycoside of Neu5Gc were also evaluated with glucose and lactose derivatives and the results suggested that the thioglycoside is a potential candidate for glycosidation as the glycosyl donor.
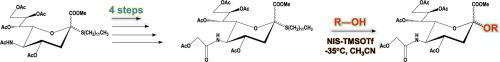
n -糖基神经氨酸(Neu5Gc)的十二烷基巯基糖苷的制备,作为装配含有Neu5Gc的低聚糖的糖基供体
以Neu5Ac的一种已知巯基苷为原料,高效合成了Neu5Gc的十二烷基巯基苷。研究了以TMSOTf-NIS为启动子体系的Neu5Gc巯基糖苷对o糖苷化的反应活性,发现该巯基糖苷对简单醇具有良好的反应活性。除了简单醇外,还对Neu5Gc的十二烷基巯基糖苷与葡萄糖和乳糖衍生物的反应性进行了评价,结果表明该巯基糖苷作为糖基供体是糖基化的潜在候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


