Catalytic Direct α-Amination of Arylacetic Acid Synthons with Anilines
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 7
Abstract
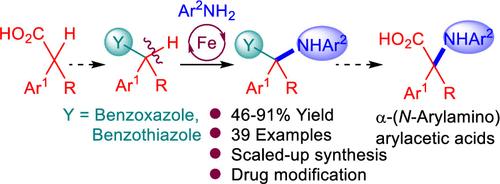
A unique α-amination approach using various anilines has been developed for arylacetic acids via adaptation as benzazoles. The reaction proceeds through a single electron transfer mechanism utilizing an iron-based catalyst system to access α-(N-arylamino)acetic acid equivalents. Modification of approved drugs, facile cleavage of the benzazole auxiliary, and tolerance of amide linkage forming conditions constitute the potential applicability of this strategy.
苯胺催化芳基乙酸合子直接α-胺化反应
采用不同的苯胺对芳基乙酸进行了独特的α-胺化反应,使其适应为苯并唑。该反应通过单电子转移机制进行,利用铁基催化剂体系获得α-(n-芳基氨基)乙酸等价物。已批准药物的修饰、苯并唑助剂的易裂解以及酰胺键形成条件的耐受性构成了该策略的潜在适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


