Development of a Scalable Enantioselective Synthesis of JAK Inhibitor Upadacitinib
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 2
Abstract
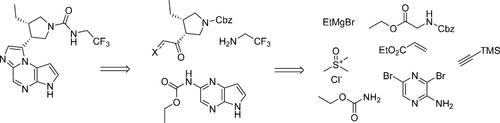
Process development of a six-stage synthesis of upadacitinib, a JAK1 inhibitor, is described. It is highlighted by an enantioselective and diastereoselective hydrogenation of a tetrasubstituted olefin to set the two pyrrolidine stereocenters. Preparation of the main fragments and strategies to link them together, optimization of the imidazole cyclization, and in-depth understanding of the formation of the urea moiety at the final stage are discussed.
可扩展对映选择性合成JAK抑制剂Upadacitinib的研究
描述了JAK1抑制剂upadacitinib的六阶段合成过程。通过四取代烯烃的对映选择性和非对映选择性加氢来设置两个吡咯烷立体中心。讨论了主要片段的制备和连接策略,咪唑环化的优化,以及对最后阶段尿素部分形成的深入了解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


