High-pressure pathways for the formation of amorphous diamond and other tetrahedrally-bonded phases from glassy carbon
IF 11.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
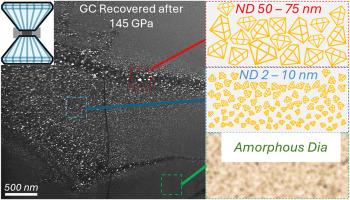
We show that non-hydrostatic compression of glassy carbon to 145 GPa at room temperature produces cubic, hexagonal and an amorphous form of diamond in the same recovered sample. The amorphous diamond phase exhibits a microstructure comparable to tetrahedral amorphous carbon thin films and to that formed from fullerene samples subjected to high pressure treatment. The crystallinity and preferred orientation of the phases indicate that shear stress is critical in driving the phase transformations from the randomly oriented graphitic glassy carbon precursor. A Gibbs free energy landscape, mapped as a function of density and crystallinity, illustrates proposed transformation pathways from glassy carbon to a common high-density parent phase that forms under compression that then converts to the phases observed, depending on the local stress environment. Modelling demonstrates that the common high-density parent phase has a preferred orientation in its atomic structure which explains the microstructure in many of the recovered daughter phases. Our results demonstrate that non-hydrostatic stress can be used to synthesise desirable diamond-like carbon phases without heating, including amorphous diamond and ultra-fine nanodiamonds with sizes down to 2 nm in diameter.
从玻璃碳中形成非晶金刚石和其他四面体键合相的高压途径
我们发现,在室温下将玻璃碳非流体静压压缩到145 GPa,在相同的回收样品中产生立方、六边形和无定形的金刚石。非晶金刚石相的微观结构类似于四面体非晶碳薄膜,也类似于富勒烯样品经高压处理后形成的非晶碳薄膜。相的结晶度和择优取向表明,剪切应力是驱动随机取向石墨玻璃碳前驱体相变的关键。吉布斯自由能图,作为密度和结晶度的函数,说明了从玻璃碳到普通高密度母相的转化途径,该母相在压缩下形成,然后根据局部应力环境转化为观察到的相。模型表明,常见的高密度母相在其原子结构上具有优先取向,这解释了许多回收的子相的微观结构。我们的研究结果表明,非流体静力应力可以在不加热的情况下合成理想的类金刚石碳相,包括非晶金刚石和直径小至2纳米的超细纳米金刚石。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


