Construction of pH-responsive dual-drug nanomedicine: Delivery of Bortezomib-Baicalein complex via bovine serum albumin nanoparticles
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
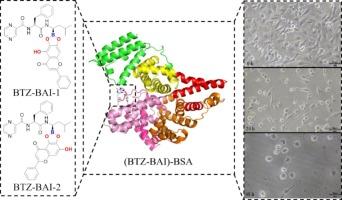
Due to the versatility and diversity, dual-drug delivery systems have become a research highlight. The boronate esters hold potential for application in developing pH-responsive nanomedicine for cancer treatment. The interaction of bortezomib (BTZ) with baicalein (BAI) to form the boronate ester BTZ-BAI was confirmed via 1H and 11B NMR spectra. Additionally, bovine serum albumin (BSA) was proved as the suitable delivery agent for BTZ-BAI. The CCK-8 test suggested that the (BTZ-BAI)-BSA complex exhibited anti-glioma effects on U251 glioma cells. Cell scratch experiments showed that (BTZ-BAI)-BSA complex also reduced the healing ability of U251 glioma cells. Fluorescent imaging experiments proved that BSA NPs could be absorbed by U251 glioma cells efficiently. These findings motivated further mechanistic investigations. The BTZ-BAI exhibited a high binding affinity to BSA at 25 °C (Ka as 2.18 × 105 L/mol). Molecular docking studies indicate that the stabilization of the (BTZ-BAI)-BSA complex is attributed to both hydrophobic forces and hydrogen bonds. These findings demonstrate that BAI does not only act as a synergistic anti-tumor agent, but also as a linker in the interaction of BTZ with BSA, which encouraged us to develop the procedure for the construction of the pH-responsive dual-drug nanomedicine (BTZ-BAI@BSA NPs).
ph响应型双药纳米药物的构建:通过牛血清白蛋白纳米颗粒递送硼替佐米-黄芩苷复合物
由于其通用性和多样性,双药给药系统已成为研究热点。硼酸酯在开发ph反应性纳米药物治疗癌症方面具有潜在的应用前景。硼替佐米(BTZ)与黄芩素(BAI)相互作用生成硼酸酯BTZ-BAI。此外,牛血清白蛋白(BSA)被证明是BTZ-BAI的合适递送剂。CCK-8实验表明(BTZ-BAI)-BSA复合物对U251胶质瘤细胞具有抗胶质瘤作用。细胞划伤实验表明,(BTZ-BAI)-BSA复合物也降低了U251胶质瘤细胞的愈合能力。荧光成像实验证明,BSA NPs能被U251胶质瘤细胞有效吸收。这些发现激发了进一步的机制研究。BTZ-BAI在25°C (Ka为2.18 × 105 L/mol)下与BSA具有较高的结合亲和力。分子对接研究表明,(BTZ-BAI)-BSA配合物的稳定性是由疏水力和氢键共同作用的结果。这些发现表明,BAI不仅是一种协同抗肿瘤药物,而且是BTZ与BSA相互作用的连接体,这鼓励了我们开发构建ph响应双药纳米药物(BTZ-BAI@BSA NPs)的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


