Microwave (MW) and ultrasound-ultraviolet (US/UV) energy-assisted green synthesis of Spiro heterocycles for high-yield efficiency and agrochemical applications
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
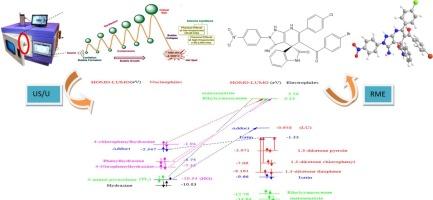
In the present work, we report on the synthesis and characterization of novel spiroheterocyclic compounds, motivated by their potential applications as pesticides. These products represent a direction of considerable scientific significance and practical value for sustainable development. To upward the green chemistry principles, efficient exploring and environmentally crucial synthesis promoting with combined microwave-ultrasonic-UV technique to facilitate the spiro synthesis via rising reaction mass efficiency, yield- and atom economy. This approach is transformed via a sono-luminescence process to create spiroheterocyclic products 5–22 in a singular synthetic endeavor. The dual US/UV system promotes efficient green parameters during work-up and minimizes waste precursors. The structural integrity of the resultant compounds is affirmed by elemental analysis, mass spectrometry, and 1H NMR and 13C NMR microanalyses. The insecticidal efficacy of the synthesized spiro derivatives was appraised against Spodoptera littoralis, Rhizoctonia solani, and Fusarium solani, with comparisons to chlorpyrifos and a creditable fungicide for certain pathogens. The structures and electronic characteristics of these novel agrochemical products were further investigated through molecular docking and density functional theory (DFT) analysis.
微波(MW)和超声-紫外(US/UV)能量辅助绿色合成螺环化合物的高产效率和农化应用
在目前的工作中,我们报告了新的螺杂环化合物的合成和表征,其潜在的应用作为农药的动机。这些产品代表了一个对可持续发展具有重要科学意义和实用价值的方向。提出绿色化学原理,利用微波-超声-紫外联合技术促进高效探索和环境关键合成,通过提高反应质量效率、产率和原子经济性来促进螺旋合成。这种方法通过声发光过程转化为在单一合成努力中创造螺杂环产品5-22。双US/UV系统在处理过程中促进了高效的绿色参数,并最大限度地减少了前驱体的浪费。通过元素分析、质谱分析、1H NMR和13C NMR微量分析证实了合成化合物的结构完整性。合成的螺旋衍生物对沿海夜蛾、茄枯丝核菌和茄枯菌的杀虫效果进行了评价,并与毒死蜱和一种可靠的杀菌剂进行了比较。通过分子对接和密度泛函理论(DFT)分析进一步研究了这些新型农化产品的结构和电子特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


