Polyaniline supported MWCNT/TiO2/Ag3PO4 nanocomposite for electrochemical determination of dopamine
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
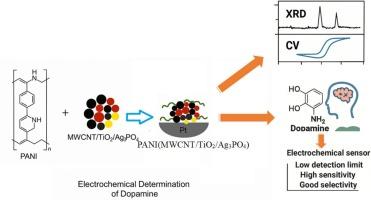
Dopamine (DA) is a neurotransmitter that plays a vital role in the brain's reward and pleasure centers which are also involved in regulating movement and emotional responses and can cause various disorders if increased or decreased from its optimal concentration. Thus, it is essential to develop rapid and simple methods for the determination of its concentration. In the present study, polyaniline-supported MWCNT/TiO2/Ag3PO4 nanocomposite was synthesized by in situ polymerization method and its structure and composition were characterized by X-ray diffraction (XRD), and Fourier transform infrared (FTIR). The electrochemical behaviors of TiO2, MWCNT, Ag3PO4, MWCNT/TiO2, TiO2/Ag3PO4, MWCNT/TiO2/Ag3PO4, and PANI(MWCNT/TiO2/ Ag3PO4) modified Pt electrodes were studied using Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS) in the presence of 2 mM K3Fe(CN)6/0.1 M KCl. The result showed that PANI-supported MWCNT/TiO2/Ag3PO4 nanocomposite was a more electroactive substrate. The same was observed towards the oxidation of dopamine due to the high electrocatalytic activity and high surface area of PANI. At the optimized condition (pH 4), the electrode was used as an electrochemical sensor for the determination of dopamine in a standard solution. The electrode exhibited a linear range (10–80, 5–120 μmol L−1), detection limits (1.51, 2.76 μmol L−1 (3σ/s) and sensitivity (4.31, 1.025 = slope/area) using Differential Pulse Voltammetry (DPV) and CV, respectively. DA followed a quasi-reversible process and its diffusion coefficient was 1.61*10−5 cm2/. The proposed sensor showed good selectivity towards DA in the presence of other electroactive compounds like ascorbic acid and is a promising DA-sensor due to its low detection limit, high sensitivity and good selectivity.
聚苯胺负载MWCNT/TiO2/Ag3PO4纳米复合材料电化学测定多巴胺
多巴胺(DA)是一种神经递质,在大脑的奖励和愉悦中心起着至关重要的作用,也参与调节运动和情绪反应,如果从最佳浓度增加或减少,会导致各种疾病。因此,开发快速简便的测定其浓度的方法是十分必要的。采用原位聚合法制备了以聚苯胺为载体的MWCNT/TiO2/Ag3PO4纳米复合材料,并用x射线衍射(XRD)和傅里叶变换红外(FTIR)对其结构和组成进行了表征。采用循环伏安法(CV)和电化学阻抗谱(EIS)研究了TiO2、MWCNT、Ag3PO4、MWCNT/TiO2、TiO2/Ag3PO4、MWCNT/TiO2/Ag3PO4和PANI(MWCNT/TiO2/ Ag3PO4)修饰Pt电极在2 mM K3Fe(CN)6/0.1 M KCl存在下的电化学行为。结果表明,聚苯胺负载的MWCNT/TiO2/Ag3PO4纳米复合材料具有更高的电活性。由于聚苯胺的高电催化活性和高表面积,对多巴胺的氧化也有同样的作用。在最佳条件下(pH为4),电极作为电化学传感器用于标准溶液中多巴胺的测定。采用差分脉冲伏安法(DPV)和CV法,电极的线性范围分别为10 ~ 80,5 ~ 120 μmol L−1,检出限分别为1.51、2.76 μmol L−1 (3σ/s),灵敏度分别为4.31、1.025 =斜率/面积。DA为准可逆过程,扩散系数为1.61*10−5 cm2/。该传感器在抗坏血酸等电活性化合物存在时对DA具有良好的选择性,检测限低、灵敏度高、选择性好,是一种很有前途的DA传感器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


