Expression pattern of the HIFα-family in equine chorioallantois during pregnancy and placental pathology
IF 2.5
2区 农林科学
Q3 REPRODUCTIVE BIOLOGY
引用次数: 0
Abstract
Equine placental dysfunction impairs fetal growth and disrupts pregnancy outcomes. Despite many advances in diagnosis, the molecular pathophysiology of equine placentitis and premature placental separation remains poorly understood. However, the hypoxia-inducible factor (HIF-α) pathway is related to placental hypoxia, and its modulation in placental diseases has been shown in other species. Therefore, we hypothesized that in conditions marked by equine placental dysfunction, oxygen deprivation in the chorioallantois membrane triggers the activation of the HIF-α pathway, which is related to molecular alterations associated with placental insufficiency. Accordingly, we compared the expression of HIF1A, HIF2A, HIF3A, and HIF1AN genes in equine chorioallantois 1) at various points during normal gestation, and in mares with 2) ascending placentitis (AP), 3) nocardioform placentitis (NP), and 4) premature placental separation (PPS), compared to mares with normal pregnancies (controls). During normal pregnancy, expression of HIF-related genes remained low until a significant post-partum increase, suggesting a balanced regulatory state under normoxic conditions. In contrast, the three pathological conditions exhibited a distinct expression profile: AP was marked by HIF1A upregulation and concurrent suppression of HIF3A and HIF1AN; NP showed reduced HIF2A and HIF3A expression without changes in HIF1A; and PPS was characterized by HIF1A upregulation with HIF1AN, HIF2A, and HIF3A downregulation. Our findings reveal that although AP, NP, and PPS all involve oxygen deprivation, the molecular hypoxia response is disease specific. This study provides novel insight into the equine placental hypoxic response. This warrants further research to elucidate these mechanisms and explore them as potential diagnostic targets for equine placental dysfunctions.
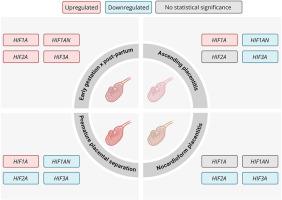
妊娠期马绒毛膜尿囊中hif α-家族的表达模式及胎盘病理。
马胎盘功能障碍损害胎儿生长和破坏妊娠结局。尽管在诊断方面取得了许多进展,但马胎盘炎和胎盘早分离的分子病理生理学仍然知之甚少。然而,缺氧诱导因子(HIF-α)途径与胎盘缺氧有关,其在胎盘疾病中的调节已在其他物种中得到证实。因此,我们假设在以马胎盘功能障碍为标志的条件下,绒毛膜尿囊膜的缺氧触发HIF-α通路的激活,这与胎盘功能不全相关的分子改变有关。因此,我们比较了HIF1A、HIF2A、HIF3A和HIF1AN基因在马绒毛膜尿囊中的表达(1)在正常妊娠的不同阶段,以及在2)升胎盘炎(AP)、3)无心状胎盘炎(NP)和4)胎盘早分离(PPS)的母马中,与正常妊娠的母马(对照)相比。在正常妊娠期间,hif相关基因的表达一直很低,直到产后显著增加,表明在正常条件下处于平衡调节状态。相比之下,三种病理状态表现出不同的表达谱:AP以HIF1A上调和HIF3A和HIF1AN同时抑制为特征;NP降低了HIF2A和HIF3A的表达,但HIF1A没有变化;PPS表现为HIF1A上调,HIF1A、HIF2A、HIF3A下调。我们的研究结果表明,尽管AP、NP和PPS都涉及缺氧,但分子缺氧反应是疾病特异性的。这项研究为马胎盘缺氧反应提供了新的见解。这需要进一步的研究来阐明这些机制,并探索它们作为马胎盘功能障碍的潜在诊断靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theriogenology
农林科学-生殖生物学
CiteScore
5.50
自引率
14.30%
发文量
387
审稿时长
72 days
期刊介绍:
Theriogenology provides an international forum for researchers, clinicians, and industry professionals in animal reproductive biology. This acclaimed journal publishes articles on a wide range of topics in reproductive and developmental biology, of domestic mammal, avian, and aquatic species as well as wild species which are the object of veterinary care in research or conservation programs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


