The fourth-generation EGFR tyrosine kinase inhibitor BLU-945 resensitizes ABCG2-overexpressing multidrug-resistant non-small cell lung cancer cells to cytotoxic anticancer drugs
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
ABCG2, a key ATP-binding cassette (ABC) transporter, contributes significantly to multidrug resistance (MDR) by actively effluxing a wide range of chemotherapeutic agents from cancer cells, thereby lowering intracellular drug concentrations and reducing therapeutic efficacy. This mechanism poses a major challenge in treating cancers such as non-small-cell lung cancer (NSCLC). Despite extensive research, no clinically approved agents specifically target ABCG2-mediated MDR. Given the success of combining tyrosine kinase inhibitors (TKIs) with chemotherapy, we explored whether BLU-945, a fourth-generation epidermal growth factor receptor (EGFR) TKI with potent activity against resistant EGFR mutations, could modulate ABCG2 function to reverse MDR. Our findings reveal that BLU-945 stimulates the ATPase activity of ABCG2, indicating direct interaction as a potential substrate or modulator. However, ABCG2-overexpressing NSCLC cells did not display resistance to BLU-945, suggesting that its antitumor activity remains intact despite high ABCG2 expression. Importantly, BLU-945 significantly resensitized these resistant cells to multiple cytotoxic agents in a concentration-dependent manner, with effects evident even at nanomolar levels. Mechanistic studies indicate that this chemosensitizing effect results from functional inhibition of ABCG2-mediated drug efflux, without affecting ABCG2 protein expression, thereby enhancing intracellular drug retention and cytotoxicity. These findings reveal a previously unrecognized pharmacological property of BLU-945 as an inhibitor of ABCG2-mediated drug efflux, supporting its potential role in combination therapies aimed at overcoming MDR in patients with ABCG2-overexpressing tumors. Further preclinical and clinical studies are warranted to validate the translational relevance of this approach and to identify patient populations that may benefit most from this combinatorial strategy.
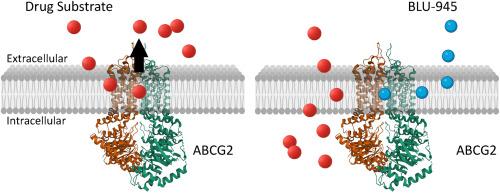
第四代EGFR酪氨酸激酶抑制剂BLU-945使过表达abcg2的多药耐药非小细胞肺癌细胞对细胞毒性抗癌药物重新敏感。
ABCG2是一种关键的atp结合盒(ABC)转运蛋白,通过从癌细胞中主动排出多种化疗药物,从而降低细胞内药物浓度,降低治疗效果,从而对多药耐药(MDR)起重要作用。这种机制对治疗非小细胞肺癌(NSCLC)等癌症提出了重大挑战。尽管广泛的研究,没有临床批准的药物专门针对abcg2介导的耐多药。鉴于酪氨酸激酶抑制剂(TKIs)联合化疗的成功,我们探索了BLU-945,第四代表皮生长因子受体(EGFR) TKI对耐药EGFR突变具有有效活性,是否可以调节ABCG2功能以逆转MDR。我们的研究结果表明,BLU-945刺激ABCG2的atp酶活性,表明其作为潜在的底物或调节剂直接相互作用。然而,ABCG2过表达的NSCLC细胞并未表现出对BLU-945的耐药性,这表明尽管ABCG2高表达,BLU-945的抗肿瘤活性仍然保持不变。重要的是,BLU-945显著地以浓度依赖的方式使这些耐药细胞对多种细胞毒性药物重新敏感,即使在纳摩尔水平上也有明显的效果。机制研究表明,这种化学增敏效应是由于ABCG2介导的药物外排的功能性抑制,而不影响ABCG2蛋白的表达,从而增强细胞内药物保留和细胞毒性。这些发现揭示了BLU-945作为abcg2介导的药物外排抑制剂的先前未被认识的药理学特性,支持其在针对abcg2过表达肿瘤患者克服耐多药耐药的联合治疗中的潜在作用。需要进一步的临床前和临床研究来验证这种方法的转化相关性,并确定可能从这种组合策略中获益最多的患者群体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


