Cooperative and stabilization effects in hydrogen-bonded chains of microhydrated thymine: a QTAIM and TD-DFT study
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We investigate the thresholds of the cooperative effects in hydrogen-bonded chains formed by thymine with 1 to 6 explicit water molecules. Using Density Functional Theory (DFT), combined with Quantum Theory of Atoms in Molecules (QTAIM) and Time-Dependent DFT (TD-DFT), we analyze the evolution of the electronic density at H-bond critical points (ρ H-bond) and its influence on the excited states. Our results indicate that the cooperative effect is stronger with the first water molecules, followed by weaker contributions beyond four water molecules. TD-DFT calculations reveal corresponding shifts in electronic transitions, linking H-bond topology with spectral changes. These findings contribute to a quantitative understanding of hydration effects in nucleobases, with implications for DNA stability and photochemistry.
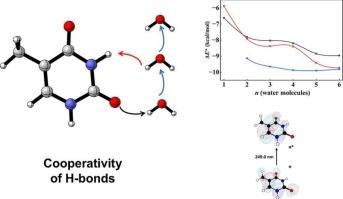
微水合胸腺嘧啶氢键链的协同和稳定效应:QTAIM和TD-DFT研究
我们研究了胸腺嘧啶与1 ~ 6个外显水分子形成的氢键链中协同效应的阈值。利用密度泛函理论(DFT),结合分子原子量子理论(QTAIM)和时变DFT (TD-DFT),分析了h键临界点(ρ h键)电子密度的演化及其对激发态的影响。结果表明,第一个水分子的协同效应较强,超过四个水分子的协同效应较弱。TD-DFT计算揭示了电子跃迁的相应位移,将氢键拓扑与光谱变化联系起来。这些发现有助于定量了解核碱基的水合作用,对DNA稳定性和光化学具有指导意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


