Crystallographic dependence of adhesion energy of chemical vapor deposition–grown monolayer graphene on copper foils
IF 11.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Chemical vapor deposition (CVD) is a widely used method for synthesizing large-area, high-quality monolayer graphene on metal substrates, particularly Cu. Although graphene transfer and manufacturing inevitably depend on interfacial interactions between graphene and seed Cu, the nature of polycrystalline Cu limits the ability to isolate the intrinsic adhesion characteristics of graphene. In this study, the effect of the crystallographic orientation of seed Cu foils on the adhesion energy of monolayer graphene was investigated through mesoscale mode I fracture tests using a double cantilever beam configuration. In addition to polycrystalline Cu foils, single-crystal Cu(111) and Cu(100) foils were prepared via an abnormal grain growth approach using high-temperature thermal annealing. Monolayer graphene was grown on those Cu foils using low-pressure CVD. Mechanical delamination of graphene from Cu revealed the adhesion energy of graphene to the underlying Cu: single-crystal Cu(111)-grown graphene exhibited a lower adhesion energy of 4.08 ± 0.68 J/m2, compared with Cu(100)-grown graphene (6.71 ± 0.86 J/m2). Polycrystalline Cu-grown graphene exhibited the highest adhesion energy (8.08 ± 0.56 J/m2), likely due to increased surface roughness caused by Cu grain boundaries. This study offers critical insights into the fundamental interfacial properties of CVD-grown graphene and highlights the importance of the seed metal selection for reliable, large-scale manufacturing of graphene-based devices.
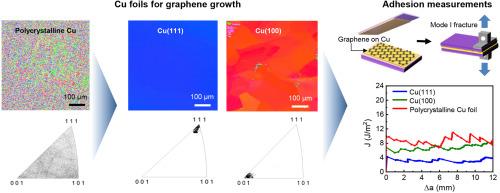
化学气相沉积单层石墨烯在铜箔上粘附能的晶体学依赖性
化学气相沉积(CVD)是一种广泛应用于在金属衬底,特别是铜上合成大面积、高质量单层石墨烯的方法。尽管石墨烯的转移和制造不可避免地依赖于石墨烯和铜种子之间的界面相互作用,但多晶铜的性质限制了分离石墨烯固有粘附特性的能力。在本研究中,通过双悬臂梁结构的中尺度I型断裂试验,研究了种子铜箔的晶体取向对单层石墨烯粘附能的影响。除制备多晶铜箔外,还采用高温退火的异常晶粒生长方法制备了单晶铜(111)和单晶铜(100)箔。利用低压气相沉积技术在铜箔上生长单层石墨烯。从Cu中机械剥离石墨烯揭示了石墨烯对底层Cu的粘附能:单晶Cu(111)生长的石墨烯表现出较低的粘附能,为4.08±0.68 J/m2,而Cu(100)生长的石墨烯为6.71±0.86 J/m2。多晶Cu生长的石墨烯表现出最高的粘附能(8.08±0.56 J/m2),这可能是由于Cu晶界增加了表面粗糙度。这项研究为cvd生长的石墨烯的基本界面特性提供了重要的见解,并强调了种子金属选择对于可靠、大规模制造石墨烯基器件的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


