How the Extent of Protein Folding and Oligomerization Modulate Condensate Formation and Properties
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Although proteins across the order–disorder continuum can undergo phase separation, it remains unclear how the structural states of the protein constituents influence the material properties of the resulting condensates. Here, using a coarse-grained model of a primordial peptide–RNA system, we investigate how condensates formed from ordered versus disordered peptides differ in their properties. By systematically varying the degree of foldedness and oligomerization of the peptide constituents, we find that stronger peptide–peptide interactions reduce diffusivity, whereas stronger peptide–RNA interactions destabilize the condensate. We further show that peptide conformational plasticity modulates the balance between these interactions, acting as a powerful lever for tuning the condensate properties. This work highlights how subtle changes in protein structure shape condensate architecture, dynamics, or stability and, together with experimental observations, provides a framework for understanding how the evolutionary shift from disordered to ordered peptides may have expanded the material repertoire of biomolecular condensates.
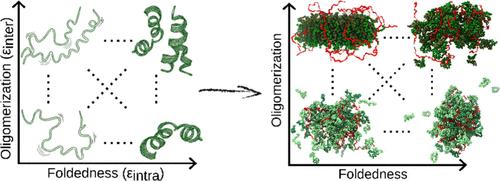
蛋白质折叠和寡聚化的程度如何调节凝析物的形成和性质
尽管有序-无序连续体中的蛋白质可以经历相分离,但仍不清楚蛋白质成分的结构状态如何影响所得凝聚物的材料特性。在这里,使用原始肽- rna系统的粗粒度模型,我们研究了由有序肽和无序肽形成的凝聚物在其性质上的差异。通过系统地改变多肽成分的折叠性和寡聚化程度,我们发现更强的肽-肽相互作用降低了扩散性,而更强的肽- rna相互作用使凝聚物不稳定。我们进一步表明,肽的构象可塑性调节了这些相互作用之间的平衡,作为调节凝聚物性质的有力杠杆。这项工作强调了蛋白质结构的细微变化如何影响凝聚物的结构、动力学或稳定性,并结合实验观察,为理解从无序肽到有序肽的进化转变如何扩大了生物分子凝聚物的材料库提供了一个框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


