Construction of hierarchical Zn-HHTP/LDH heterostructures for selective and enhanced lithium extraction via capacitive deionization
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Efficient and sustainable lithium extraction from aqueous resources is vital for advancing next-generation energy storage technologies. However, conventional LiAl-layered double hydroxide (LDH) adsorbents are hindered by low adsorption capacity, sluggish ion transport, and poor regeneration. Here, we present a hierarchically engineered Zn-HHTP/LDH heterostructured electrode, prepared by Zn doping into LiAl-LDH to form LiZnAl-LDH on carbon cloth, followed by in situ coordination with 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) to construct a conductive metal–organic framework (c-MOF). The resulting -d conjugated interface significantly enhances charge transport, accelerates Li diffusion, and increases the density of active sites. When applied in a capacitive deionization (CDI) system, the electrode delivers a high Li adsorption capacity (50.25 mg g), excellent specific capacitance (198.33 F g in 10 mM LiCl), and superior cycling stability (87.5% capacity retention after 100 cycles). In complex brines, it achieves exceptional selectivity (Li/Mg = 17.75). Density functional theory and molecular dynamics simulations reveal that the enhanced performance originates from interfacial charge redistribution, a reduced Li migration barrier (0.24 eV), and robust Zn-O coordination. This work offers a promising CDI platform and provides mechanistic insights into the rational design of heterostructured electrodes for efficient and selective lithium recovery from challenging aqueous environments.
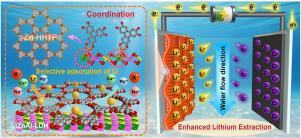
Zn-HHTP/LDH层次化异质结构的构建及其在电容去离子化锂萃取中的应用
高效、可持续地从水中提取锂对于推进下一代储能技术至关重要。然而,传统的lial层状双氢氧化物(LDH)吸附剂存在吸附能力低、离子传输缓慢、再生能力差等问题。在此,我们提出了一种分层工程的Zn-HHTP/LDH异质结构电极,将Zn掺杂到LiAl-LDH中,在碳布上形成LiZnAl-LDH,然后与2,3,6,7,10,11-六羟基三苯(HHTP)原位配位,构建导电金属-有机框架(c-MOF)。由此产生的π-d共轭界面显著增强了电荷输运,加速了Li+的扩散,并增加了活性位点的密度。当应用于电容去离子(CDI)系统时,电极提供了高Li+吸附容量(50.25 mg g−1),优异的比电容(198.33 F g−1在10 mM LiCl中)和优越的循环稳定性(100次循环后容量保持率为87.5%)。在复杂的盐水中,它具有优异的选择性(Li+/Mg2+ = 17.75)。密度泛函理论和分子动力学模拟表明,性能的增强源于界面电荷重分布、Li+迁移势垒降低(0.24 eV)和Zn-O4配位的增强。这项工作提供了一个很有前途的CDI平台,并为异质结构电极的合理设计提供了机制见解,以便从具有挑战性的水环境中高效和选择性地回收锂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


