Production of battery grade power materials from spent lithium iron phosphate (LiFePO4) batteries
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Lithium iron phosphate (LiFePO4) batteries have gained popularity due to their high safety and low cost. Effective recycling processes are needed to sustain indigenous material and economic benefit. In other hand, power materials are imported for the manufacturing of batteries, which compells for the development of process for recovery of the valuable metals (Fe, P, Li, Cu, and Al) from spent LFP batteries. Leaching was carried out at optimized condition using 3 % H2SO4 and 5 % H2O2 maintaining 100 g/L pulp density at 60 °C. 99.99 % Fe, Li, P, Al, and Cu were recovered within 60 min of mixing time. Leaching kinetics of Li followed “Chemical reaction control dense constant size cylindrical particles” model 1−(1−X)1/2 = kct, while Fe, P, Al, and Cu adhered to a “Chemical reaction control dense constant size or shrinking spheres” model 1−(1−X)1/3 = kct. Cementation was carried out using scrap iron to recover Cu at room temperature within 40 min. Fe and P were recovered as FePO4 by precipitation. 99.99 % Al was precipitated using NaOH at pH 7.5, whereas 99.9 % Li was precipitated as Li2CO3 and Li3PO4 at pH 12 and 90 °C using Na2CO3 and Na3PO4.12H2O, respectively. This process is viable for recycling LFP batteries ensuring resource recovery and environmental sustainability.
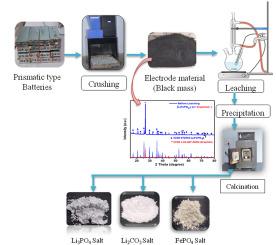
利用废旧磷酸铁锂(LiFePO4)电池生产电池级动力材料
磷酸铁锂(LiFePO4)电池因其高安全性和低成本而受到欢迎。需要有效的回收过程来维持本地材料和经济效益。另一方面,用于制造电池的动力材料是进口的,这促使了从废LFP电池中回收有价值金属(铁、磷、锂、铜和铝)的工艺的发展。在最佳浸出条件下,采用3% H2SO4和5% H2O2,浸出温度为60℃,矿浆密度为100 g/L,混合时间为60 min,可回收99.99%的Fe、Li、P、Al和Cu。Li浸出动力学遵循“化学反应控制致密恒尺寸柱状颗粒”模型1−(1−X)1/2 = kct, Fe、P、Al和Cu浸出动力学遵循“化学反应控制致密恒尺寸或收缩球”模型1−(1−X)1/3 = kct。利用废铁在室温下40 min内进行胶结回收Cu。Fe和P通过沉淀回收为FePO4。用Na2CO3和Na3PO4.12H2O在pH为12和90°C的条件下分别以Li2CO3和Li3PO4的形式沉淀99.9%的Li。该工艺对于LFP电池的回收是可行的,确保了资源的回收和环境的可持续性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


