The importance of non-ideality of protons and water in the open circuit potential modelling of redox flow battery aqueous electrolytes
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The non-ideality of electrolytes is commonly neglected in redox flow battery (RFB) modelling work. The neglect of activity coefficients introduces a discrepancy of up to 100 mV between the experimental open circuit potential (OCP) and model predicted reversible Nernstian potential, which propagates into inaccurate state of charge estimation and misinterpretation of thermodynamic efficiency. Due to the complex chemistry and the lack of thermodynamic data, activity coefficients of individual species in highly non-ideal electrolytes are often difficult to determine. In this work, proton and water activity values were calculated using geochemical numerical codes implementing the Pitzer theory, a semi-empirical thermodynamic model that accounts for the non-ideal behavior of ions in electrolyte solutions. The completed Nernst equation with corrected activity values has been elaborated for OCP comparison. An in-operando approach was proposed to measure the proton and water activities of strongly acidic electrolytes. The cell OCP of Hydrogen-Vanadium RFB (H2-V RFB) and Hydrogen-Manganese RFB (H2-Mn RFB) was measured and calculated to assess the electrochemical significance of proton and water non-ideality to the RFB model. With the measured activity data, the error between the experimental and calculated OCP values was reduced from 88-100 mV to 17–50 mV.
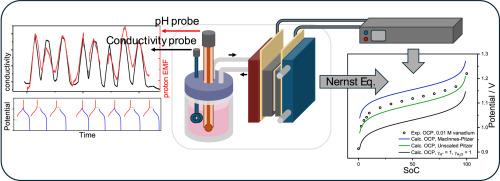
质子和水的非理想性在氧化还原液流电池开路电位建模中的重要性
在氧化还原液流电池(RFB)的建模工作中,电解质的非理想性通常被忽略。活度系数的忽略导致实验开路电位(OCP)与模型预测的可逆能势之间存在高达100 mV的差异,从而导致不准确的电荷状态估计和热力学效率的误解。由于复杂的化学性质和热力学数据的缺乏,高度非理想电解质中单个物质的活度系数往往难以确定。在这项工作中,质子和水的活度值是使用地球化学数值代码实现Pitzer理论计算的,Pitzer理论是一种半经验热力学模型,用于解释电解质溶液中离子的非理想行为。经修正活度值后的完整能斯特方程已被详细阐述用于OCP比较。提出了一种测量强酸性电解质质子和水活度的方法。测量并计算氢钒RFB (H2-V RFB)和氢锰RFB (H2-Mn RFB)的电池OCP,以评估质子和水非理想性对RFB模型的电化学意义。利用实测活度数据,将实验值与计算值之间的误差从88 ~ 100 mV减小到17 ~ 50 mV。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


