Biomimetic membrane-coating zinc sulfide nanoparticles for anti-inflammatory combined neuroprotective therapy for spinal cord injury
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Spinal cord regeneration remains challenging due to complex inflammatory microenvironments, imbalances in metal ions, and obstacles to neuronal regeneration following spinal cord injury (SCI). Herein, microglial cell membranes coated with zinc sulfide nanoparticles modified with albumin (ZnS@BSA@MM) were designed as an anti-inflammatory combined neuroprotective therapy for SCI. ZnS@BSA@MM NPs were constructed via albumin modification and membrane extrusion and exhibited ROS-scavenging abilities comparable to those of natural products and slow H2S release under acidic conditions. In vitro and in vivo experiments demonstrated the outstanding therapeutic effects of the ZnS@BSA@MM. In detail, the released H2S and Zn2+ not only inhibit microglial activation through the NF-κB signaling axis but also promote the axonal growth of neurons under pathological conditions. Notably, microglial cell membranes effectively deliver ZnS@BSA to the lesion area. Finally, ZnS@BSA@MM facilitated the axonal regeneration of neurons in SCI, suppressed inflammatory responses, and activated multiple pathways, including cytokine-cytokine receptor interactions, neuroactive ligand-receptor interactions, and cAMP signaling. Collectively, this work highlights the anti-inflammatory and neuroprotective effects of ZnS@BSA@MM NPs, featuring satisfactory H2S release and Zn2+ supplementation under membrane-targeting conditions for SCI therapy.
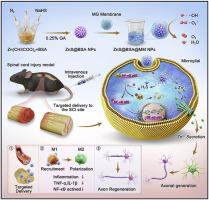
仿生膜包覆硫化锌纳米颗粒抗炎联合神经保护治疗脊髓损伤
由于复杂的炎症微环境、金属离子失衡以及脊髓损伤(SCI)后神经元再生障碍,脊髓再生仍然具有挑战性。本研究设计了以白蛋白修饰的硫化锌纳米颗粒(ZnS@BSA@MM)包被的小胶质细胞膜作为抗炎联合神经保护治疗脊髓损伤的方法。ZnS@BSA@MM NPs通过白蛋白修饰和膜挤压构建,具有与天然产物相当的ros清除能力,并且在酸性条件下缓慢释放H2S。体外和体内实验证明ZnS@BSA@MM具有显著的治疗效果。在病理条件下,释放的H2S和Zn2+不仅通过NF-κB信号轴抑制小胶质细胞的激活,而且促进神经元轴突的生长。值得注意的是,小胶质细胞膜有效地将ZnS@BSA传递到病变区域。最后,ZnS@BSA@MM促进脊髓损伤神经元轴突再生,抑制炎症反应,激活多种途径,包括细胞因子-细胞因子受体相互作用,神经活性配体-受体相互作用和cAMP信号传导。总的来说,这项工作强调了ZnS@BSA@MM NPs的抗炎和神经保护作用,在膜靶向条件下具有令人满意的H2S释放和Zn2+补充,用于脊髓损伤治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


