Effect of Nb and Y co-doping on the layered perovskite cathode of protonic ceramic fuel cells
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
To address the performance bottleneck of protonic ceramic fuel cells (PCFCs) associated with sluggish oxygen reduction reaction (ORR) kinetics, this study explores the co-doping effect of Nb and Y in layered perovskite oxides to develop an efficient and stable cathode. A series of PrBa0.9Co1.96NbxY0.04-xO5+δ (x = 0, 0.01, 0.02, 0.03, and 0.04) cathode materials were synthesized via a sol-gel method and systematically evaluated. Among them, the optimized cathode material PrBa0.9Co1.96Nb0.01Y0.03O5+δ with x = 0.01 (PBCNb0.01Y0.03) demonstrates enhanced surface oxygen vacancy concentration, higher Co4+ content, and improved catalytic ORR activity. The PBCNb0.01Y0.03 cathode exhibits a reduced polarization resistance across 700–550 °C compared to its counterparts. Co-doping of Nb and Y optimizes the processes of charge transfer, oxygen adsorption/dissociation, and ion migration, as suggested by the distribution of relaxation time (DRT) analysis. A peak power density of 2.23 W cm−2 is achieved at 700 °C from the single cell with PBCNb0.01Y0.03 cathode, outperforming most reported cathodes with similar architectures. Additionally, a promising electrochemical stability over extended operation (100 h) is demonstrated at 0.5 A cm−2.
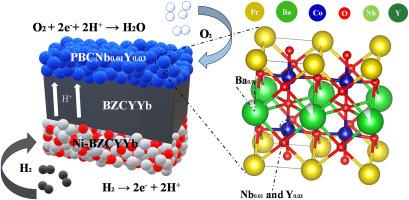
铌钇共掺杂对质子陶瓷燃料电池层状钙钛矿阴极的影响
为了解决质子陶瓷燃料电池(pcfc)与氧还原反应(ORR)动力学缓慢相关的性能瓶颈,本研究探索了铌和Y在层状钙钛矿氧化物中的共掺杂效应,以开发一种高效稳定的阴极。采用溶胶-凝胶法制备了一系列PrBa0.9Co1.96NbxY0.04-xO5+δ (x = 0、0.01、0.02、0.03和0.04)正极材料,并对其进行了系统评价。其中,优化后的正极材料prba0.9 co1.96 nb0.01 y0.030 o5 +δ, x = 0.01 (PBCNb0.01Y0.03),表面氧空位浓度增强,Co4+含量提高,催化ORR活性提高。与同类材料相比,PBCNb0.01Y0.03阴极在700-550°C范围内的极化电阻降低。根据弛豫时间(DRT)分布分析,Nb和Y的共掺杂优化了电荷转移、氧吸附/解离和离子迁移过程。使用PBCNb0.01Y0.03阴极的单电池在700°C下实现了2.23 W cm−2的峰值功率密度,优于大多数具有类似结构的阴极。此外,在0.5 a cm−2下,延长操作(100 h)的电化学稳定性很有希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


