Dual-specificity protein phosphatase 1: A potential therapeutic target in cancer
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
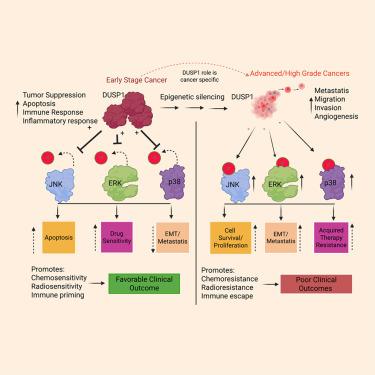
Dual-specificity protein phosphatase 1 (DUSP1), also known as MAP kinase phosphatase 1 (MKP1), is a key member of the dual-specificity phosphatase family that dephosphorylates both threonine and tyrosine residues on mitogen-activated protein kinases (MAPKs). By inactivating critical MAPK signaling pathways, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, DUSP1 serves as a pivotal regulator of diverse cellular processes such as proliferation, differentiation, apoptosis, autophagy, and stress responses. Emerging evidence highlights its context-dependent roles in cancer progression, where DUSP1 can function either as a tumor suppressor or promoter depending on the tumor type, stage, and tumor microenvironment (TME). Aberrant DUSP1 regulation is implicated in modulating cancer cell resistance to chemotherapy and radiotherapy, as well as facilitating immune evasion within the TME. This review provides a comprehensive overview of DUSP1, including molecular, structural, and regulatory mechanisms; its role in tumorigenesis, drug resistance, and immune modulation; and its therapeutic potential in precision oncology.
双特异性蛋白磷酸酶1:癌症的潜在治疗靶点
双特异性蛋白磷酸酶1 (DUSP1),也称为MAP激酶磷酸酶1 (MKP1),是双特异性磷酸酶家族的关键成员,可使丝裂原活化蛋白激酶(MAPKs)上的苏氨酸和酪氨酸残基去磷酸化。DUSP1通过灭活关键的MAPK信号通路,包括细胞外信号调节激酶(ERK)、c-Jun n -末端激酶(JNK)和p38,作为多种细胞过程的关键调节剂,如增殖、分化、凋亡、自噬和应激反应。新出现的证据强调了它在癌症进展中的环境依赖性作用,其中DUSP1可以根据肿瘤类型、分期和肿瘤微环境(TME)作为肿瘤抑制因子或启动子发挥作用。异常的DUSP1调控涉及调节癌细胞对化疗和放疗的耐药性,以及促进TME内的免疫逃避。本文综述了DUSP1的分子、结构和调控机制;它在肿瘤发生、耐药和免疫调节中的作用;以及它在精准肿瘤学方面的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


