PEA15 promotes osteosarcoma progression and cisplatin resistance by modulating autophagy through the FABP3-TNF signaling axis
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Osteosarcoma (OS), a primary malignant bone tumor, is characterized by resistance to chemotherapeutic agents such as cisplatin (DDP), posing a major obstacle to effective treatment. Tumor cells often exploit autophagy to survive chemotherapeutic stress, which contributes to this resistance. Using weighted gene co-expression network analysis (WGCNA), this study screened for autophagy-related genes associated with OS prognosis and identified PEA15 as a key indicator of poor outcomes. Through gene knockdown and overexpression experiments in OS cell lines and xenograft models, we found that PEA15 promotes tumor progression. Mechanistically, RNA sequencing revealed that PEA15 inhibits autophagy and apoptosis by modulating the downstream target FABP3 and the associated TNF signaling pathway. Notably, silencing PEA15 in resistant OS cells enhanced their sensitivity to cisplatin by activating autophagy. These findings identify the PEA15-FABP3-TNF signaling axis as a key pathway regulating chemoresistance in OS, suggesting that targeting PEA15 could be a promising therapeutic strategy to improve patient outcomes.
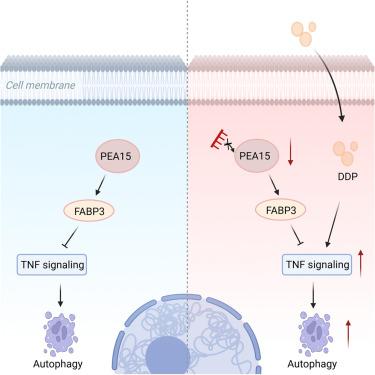
PEA15通过FABP3-TNF信号轴调节自噬,促进骨肉瘤进展和顺铂耐药
骨肉瘤(Osteosarcoma, OS)是一种原发性骨恶性肿瘤,其特点是对顺铂(DDP)等化疗药物具有耐药性,是有效治疗的主要障碍。肿瘤细胞经常利用自噬来生存化疗应激,这有助于这种抵抗。本研究利用加权基因共表达网络分析(WGCNA)筛选与OS预后相关的自噬相关基因,并将PEA15确定为预后不良的关键指标。通过在OS细胞系和异种移植瘤模型中进行基因敲除和过表达实验,我们发现PEA15促进肿瘤进展。在机制上,RNA测序显示PEA15通过调节下游靶点FABP3和相关的TNF信号通路抑制自噬和凋亡。值得注意的是,在耐药OS细胞中沉默PEA15可通过激活自噬增强其对顺铂的敏感性。这些发现确定PEA15- fabp3 - tnf信号轴是调节OS化疗耐药的关键途径,表明靶向PEA15可能是改善患者预后的有希望的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


