Comparative evaluation of chemical and UV mutagenesis with post-mutagenesis process optimization for enhanced cellulolytic enzyme production by Aspergillus oryzae NCIM 637
Q1 Environmental Science
引用次数: 0
Abstract
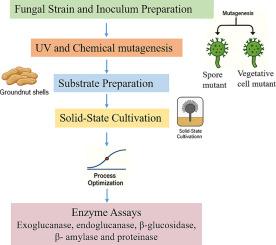
This study evaluated the effects of chemical and UV mutagenesis on Aspergillus oryzae NCIM 637 to enhance cellulolytic enzyme production under solid-state fermentation (SSF) using groundnut shells. Chemical mutagenesis has produced the most consistent improvements. The chemically treated vegetative cell mutant M4 showed a 58.7 % increase in metabolic activity and substantial gains in enzyme yields: endoglucanase (222.5 IU/gDW, 77.9 %), exoglucanase (238 IU/gDW, 62.5 %), β-glucosidase (560.7 IU/gDW, 61.9 %), β-xylanase (652.8 IU/gDW, 55.4 %), β-amylase (221.4 IU/gDW, 48.7 %), and proteinase (196.6 IU/gDW, 2.1-fold over WT). UV mutagenesis has been shown to elicit more selective responses than chemical mutagenesis. The vegetative cell mutant M1 showed the highest improvement, with an 85.3 % increase in metabolic activity, alongside elevated β-xylanase and proteinase activities. Statistical analysis confirmed the significant effects of mutagen type and incubation time (p < 0.001). RSM modeling provided strong predictive fits (R2 = 0.93–0.97) with desirability indices of >0.95. Process optimization further increased the yields, particularly for β-xylanase (70.1 %) and endoglucanase (61.96 %). Overall, chemical mutagenesis generated broad-spectrum and stable improvements, whereas UV irradiation selectively stimulated specific enzymatic pathways. These results demonstrate a scalable approach for valorizing agro-residues through optimized fungal mutagenesis and SSF, advancing sustainable enzyme production for industrial applications.
米曲霉NCIM 637的化学诱变与紫外诱变的比较评价及诱变后工艺优化
本研究评价了化学诱变和紫外诱变对米曲霉NCIM 637的影响,以提高花生壳固态发酵(SSF)下纤维素水解酶的产量。化学诱变产生了最一致的改进。经化学处理的营养细胞突变体M4代谢活性增加58.7%,酶产量大幅增加:内切葡聚糖酶(222.5 IU/gDW, 77.9%)、外葡聚糖酶(238 IU/gDW, 62.5%)、β-葡萄糖苷酶(560.7 IU/gDW, 61.9%)、β-木聚糖酶(652.8 IU/gDW, 55.4%)、β-淀粉酶(221.4 IU/gDW, 48.7%)和蛋白酶(196.6 IU/gDW,比WT高2.1倍)。紫外线诱变已被证明比化学诱变引起更多的选择性反应。营养细胞突变体M1表现出最大的改善,代谢活性提高了85.3%,同时β-木聚糖酶和蛋白酶活性也有所提高。统计分析证实诱变剂类型和孵育时间有显著影响(p < 0.001)。RSM模型提供了较强的预测拟合(R2 = 0.93-0.97),期望指数为>;0.95。工艺优化进一步提高了β-木聚糖酶(70.1%)和内切葡聚糖酶(61.96%)的收率。总的来说,化学诱变产生了广谱和稳定的改善,而紫外线照射选择性地刺激了特定的酶途径。这些结果展示了一种可扩展的方法,通过优化真菌诱变和SSF来实现农业残留物的增值,促进了可持续酶生产的工业应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioresource Technology Reports
Environmental Science-Environmental Engineering
CiteScore
7.20
自引率
0.00%
发文量
390
审稿时长
28 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


