Dimers involving methane: Strength, range, and nature of the intermolecular interaction and their thermodynamic stability in gas-phase
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
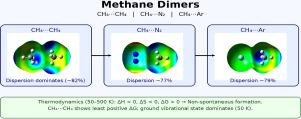
This work focuses on an accurate theoretical characterization of the strength, range, and nature of the weak intermolecular interaction that controls the formation of CH ![]() CH, CH
CH, CH ![]() N, and CH
N, and CH ![]() Ar gaseous dimers. It also provides a reliable valuation of the thermodynamic stability of such three adducts and of their relative distribution of populated intermolecular vibrational levels. The formation of these dimers is driven by the negative components of enthalpy and entropy, indicating that these complexes form nonspontaneously through gas-phase collisions. The results also suggest that the main interaction contribution controlling the stability of these adducts is the dispersion attraction. Furthermore, the formation of the CH
Ar gaseous dimers. It also provides a reliable valuation of the thermodynamic stability of such three adducts and of their relative distribution of populated intermolecular vibrational levels. The formation of these dimers is driven by the negative components of enthalpy and entropy, indicating that these complexes form nonspontaneously through gas-phase collisions. The results also suggest that the main interaction contribution controlling the stability of these adducts is the dispersion attraction. Furthermore, the formation of the CH ![]() CH complex, driven by the stronger interaction, exhibits the least positive free energy variation over a wide range of considered temperatures. The obtained information is of relevance since it opens up a range of applications in several areas, including chemistry, materials science, and astrophysics.
CH complex, driven by the stronger interaction, exhibits the least positive free energy variation over a wide range of considered temperatures. The obtained information is of relevance since it opens up a range of applications in several areas, including chemistry, materials science, and astrophysics.
含甲烷二聚体:分子间相互作用的强度、范围和性质及其在气相中的热力学稳定性
这项工作的重点是对控制CH4 CH4, CH4 N2和CH4 Ar气体二聚体形成的弱分子间相互作用的强度,范围和性质的准确理论表征。它还提供了这三种加合物的热力学稳定性及其密集分子间振动水平的相对分布的可靠评价。这些二聚体的形成是由负的焓和熵驱动的,表明这些配合物是通过气相碰撞非自发形成的。结果还表明,控制这些加合物稳定性的主要相互作用贡献是分散吸引。此外,在较强相互作用的驱动下,CH4 - CH4络合物的形成在广泛的温度范围内表现出最小的正自由能变化。获得的信息是相关的,因为它在几个领域开辟了一系列的应用,包括化学、材料科学和天体物理学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


