What does the “Trojan horse” carry? Effects of perfluorooctanoic acid on the migration and transformation of sulfur during dyeing sludge incineration
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Dyeing sludge (DS) serves as a high-capacity carrier for both conventional pollutants (e.g., organic sulfur compounds) and emerging contaminants such as per- and polyfluoroalkyl substances (PFASs). However, how PFASs influence the thermal transformation of organic sulfur during DS incineration remains poorly understood. This study investigated the effects of perfluorooctanoic acid (PFOA), a representative PFASs, on the combustion behavior of model organic sulfur compounds—sulfone (S) and mercaptan (M). The combustion of S/M–PFOA mixtures was primarily governed by the organic sulfur species, with PFOA reducing the heat threshold required for sustained combustion. The presence of PFOA enhanced the mineralization of organic sulfur, leading to significant emissions of SO₂, CH₃SH, and HF. The yields of CH₃SH and SO₂ from the mixtures increased by 1.11–5.58 and 1.30–6.34 times, respectively, compared with the organic sulfur alone, attributable to a “Trojan Horse” effect involving coupled radical and secondary reactions. PFOA lowered the binding energies of the mixtures by 26.55–62.57%, effectively reducing the combustion barrier of organic sulfur. The low formation energies of S–PFOA (–872.21 kcal/mol) and M–PFOA (–729.70 kcal/mol) confirmed the spontaneous and synergistic combustion of PFAS–sulfur mixtures. Overall, PFASs act as a “Trojan horse,” intensifying sulfur-related emissions during DS incineration. Understanding PFAS–organic sulfur interactions across multiphase systems is crucial for mitigating secondary environmental risks during thermal treatment.
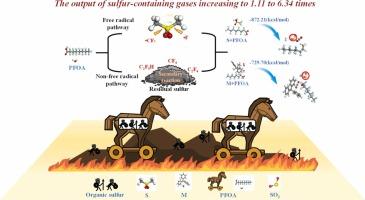
“特洛伊木马”携带的是什么?全氟辛酸对印染污泥焚烧过程中硫迁移转化的影响
染色污泥(DS)是传统污染物(如有机硫化合物)和新兴污染物(如全氟烷基和多氟烷基物质)的高容量载体。然而,在DS焚烧过程中,PFASs如何影响有机硫的热转化仍然知之甚少。本研究考察了全氟辛酸(PFOA)对模型有机硫化合物砜(S)和硫醇(M)燃烧行为的影响。S/ M-PFOA混合物的燃烧主要受有机硫的控制,PFOA降低了持续燃烧所需的热阈值。PFOA的存在增强了有机硫的矿化,导致so2、CH₃SH和HF的显著排放。与单独使用有机硫相比,混合物中CH₃SH和SO₂的产率分别提高了1.11-5.58倍和1.30-6.34倍,这是由于自由基和次级反应耦合的“特洛伊木马”效应。PFOA使混合物结合能降低26.55 ~ 62.57%,有效降低了有机硫的燃烧阻隔。S-PFOA (-872.21 kcal/mol)和M-PFOA (-729.70 kcal/mol)较低的生成能证实了pfa -硫混合物的自燃和协同燃烧。总的来说,全氟辛烷磺酸就像一个“特洛伊木马”,在DS焚烧过程中加剧了与硫有关的排放。了解pfas -有机硫在多相系统中的相互作用对于减轻热处理过程中的二次环境风险至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


