Triple-Loop Dynamic DNA Nanonetwork with Cascaded Signal Amplification toward Self-Validating MicroRNA Biosensing
IF 3.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
MicroRNAs, critical mediators of epigenetic regulation, govern vital biological processes with dysregulation linked to diseases, yet their low abundance and high sequence homology impose dual challenges on detection sensitivity and specificity. To overcome these limitations, a fluorescence and photoelectrochemical dual-mode biosensor was engineered, employing an entropy-driven circuit (EDC) and DNAzyme-based triple-loop cascade nanonetwork. Target recognition initiates EDC-mediated release of the resulting products, simultaneously activating DNAzyme I and DNAzyme II to produce target analogues (T*), thereby establishing a dual-amplification loop in which T* triggers EDC feedback to form a cascaded amplification nanonetwork. Signal transduction was enabled by CdS quantum dots (QDs), with the response synergistically amplified by g-C3N4 nanosheets to achieve enhanced photoelectrochemical performance. Furthermore, the elaborately designed positive feedback triple amplification system substantially improves the sensor's detection sensitivity. Specifically, the EDC amplification module maximizes the utilization of DNA strands, thereby significantly enhancing both operational efficiency and cost-effectiveness. Additionally, the precisely engineered triple amplification nanonetwork combined with magnetic extraction substantially improves the sensor's resistance to interference from potential coexisting substances, thereby enhancing its specificity and stability. Moreover, the dual-signal output mechanism in this design enables mutual validation of the detection results, further reinforcing the reliability and credibility of the assay outcomes.
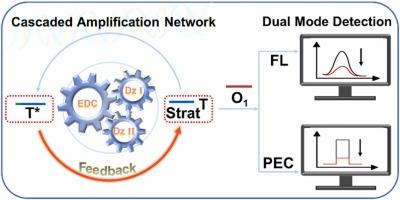
具有级联信号放大的三环动态DNA纳米网络用于自我验证的MicroRNA生物传感
microrna是表观遗传调控的关键介质,控制着与疾病相关的重要生物过程的失调,但其低丰度和高序列同源性对检测灵敏度和特异性提出了双重挑战。为了克服这些限制,设计了一种荧光和光电化学双模生物传感器,采用熵驱动电路(EDC)和基于dnazyme的三回路级联纳米网络。目标识别启动EDC介导的产物释放,同时激活DNAzyme I和DNAzyme II产生目标类似物(T*),从而建立一个双扩增环路,其中T*触发EDC反馈形成级联扩增纳米网络。通过CdS量子点(QDs)实现信号转导,并通过g-C3N4纳米片协同放大响应,从而增强光电化学性能。此外,精心设计的正反馈三倍放大系统大大提高了传感器的检测灵敏度。具体来说,EDC扩增模块最大限度地利用DNA链,从而显著提高操作效率和成本效益。此外,精确设计的三重放大纳米网络结合磁提取,大大提高了传感器对潜在共存物质干扰的抵抗力,从而增强了其特异性和稳定性。此外,本设计的双信号输出机制使检测结果能够相互验证,进一步增强了检测结果的可靠性和可信度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


