Comparative analysis of high-intensity non-thermal physical treatments on the structural and functional properties of Spirulina platensis protein
IF 9.7
1区 化学
Q1 ACOUSTICS
引用次数: 0
Abstract
Spirulina platensis protein (SPP) has attracted attention as a sustainable alternative to conventional proteins. However, its structural and functional performance under different processing conditions remains underexplored. This study investigated the effects of three non-thermal physical treatments—high hydrostatic pressure (HHP, 100–600 MPa), high-intensity ultrasound (HIU, 70–100 % amplitude), and high-speed shear homogenization (HSS, 1.0–2.5 W)—on the structure and functionality of SPP. SDS-PAGE revealed treatment-specific alterations in protein subunits, accompanied by distinct changes in sulfhydryl/disulfide, surface hydrophobicity, intrinsic fluorescence, and secondary structure. HIU induced the strongest structural disruption, characterized by reduced α-helix content, exposure of hydrophobic residues, and a pronounced decrease in particle size and ζ-potential, whereas HHP promoted unfolding at moderate pressures but aggregation at higher levels. HSS primarily reduced particle size with moderate effects on unfolding. Collectively, these results indicate that high-Intensity non-Thermal physical treatments effectively modulate the functional properties of SPP, demonstrating its potential as a protein ingredient for food applications.
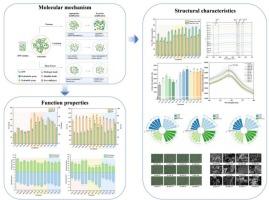
高强度非热物理处理对螺旋藻蛋白结构和功能特性的影响。
螺旋藻蛋白(SPP)作为一种可持续的蛋白质替代品受到了广泛的关注。然而,其在不同加工条件下的结构和功能性能仍有待进一步研究。本研究研究了三种非热物理处理——高静水压力(HHP, 100-600 MPa)、高强度超声(HIU, 70- 100%振幅)和高速剪切均质(HSS, 1.0-2.5 W)对SPP结构和功能的影响。SDS-PAGE显示了蛋白质亚基的特异性改变,伴随着巯基/二硫、表面疏水性、固有荧光和二级结构的明显变化。HIU诱导了最强烈的结构破坏,其特征是α-螺旋含量降低,疏水残基暴露,颗粒大小和ζ-电位明显降低,而HHP在中等压力下促进了展开,但在较高压力下促进了聚集。高速钢主要降低了颗粒尺寸,对展开有中等影响。总之,这些结果表明,高强度的非热物理处理有效地调节了SPP的功能特性,表明了其作为食品中蛋白质成分的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Ultrasonics Sonochemistry
化学-化学综合
CiteScore
15.80
自引率
11.90%
发文量
361
审稿时长
59 days
期刊介绍:
Ultrasonics Sonochemistry stands as a premier international journal dedicated to the publication of high-quality research articles primarily focusing on chemical reactions and reactors induced by ultrasonic waves, known as sonochemistry. Beyond chemical reactions, the journal also welcomes contributions related to cavitation-induced events and processing, including sonoluminescence, and the transformation of materials on chemical, physical, and biological levels.
Since its inception in 1994, Ultrasonics Sonochemistry has consistently maintained a top ranking in the "Acoustics" category, reflecting its esteemed reputation in the field. The journal publishes exceptional papers covering various areas of ultrasonics and sonochemistry. Its contributions are highly regarded by both academia and industry stakeholders, demonstrating its relevance and impact in advancing research and innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


