Metallosalphen-Covalent Organic Framework-Based Semiconducting Artificial Enzymes with Radio-Activable Antitumor Immunity for Suppressing Tumor Metastasis and Recurrence
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Reactive oxygen species (ROS)-catalytic therapies have gained increasing popularity in preventing tumor metastasis and recurrence, yet their efficiency is often compromised by limited systemic immune activation. Herein, we report the de novo design of Ru-coordinated bis-Schiff base salphen-covalent organic frameworks (SCOF-Ru) to serve as semiconducting artificial enzymes with radio-activable ROS-catalytic efficiency and antitumor immunity for suppressing tumor metastasis and recurrence. Experimental and theoretical results demonstrate that the semiconducting SCOF-Ru displays large π-conjugation, efficient electron transfer, strong electron–hole separation, and unique Ru2–N4O2 catalytic centers, enabling the most superior ROS production capability under low-dose X-ray irradiation. Rather than relying on high-Z elements, semiconducting SCOF-Ru with optimized band structures endows Ru sites with high radiosensitization effects. Our findings have disclosed that the SCOF-Ru can not only effectively inhibit DNA repair but also trigger robust apoptosis through the downregulation of calcium signaling pathways. Correspondingly, the therapeutic superiority and recurrence inhibition efficacies of SCOF-Ru have been validated in different tumor models, especially radiotherapy-resistant patient-derived xenograft models. Combined with immune checkpoint blockade, radio-activable SCOF-Ru shows great potential to robustly inhibit the growth of distant tumors. We believe the innovative design of ROS-catalytic and radio-activable artificial enzymes will enable a promising avenue for treating malignant tumors.
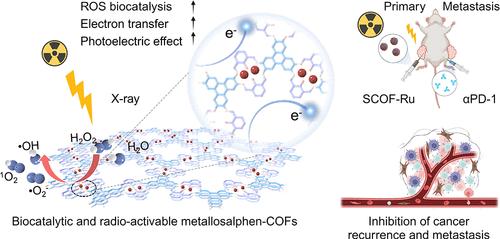
基于金属沙芬共价有机骨架的半导体人工酶,具有可放射激活的抗肿瘤免疫抑制肿瘤转移和复发
活性氧(ROS)催化疗法在预防肿瘤转移和复发方面越来越受欢迎,但其效率往往受到有限的全身免疫激活的影响。在此,我们报道了钌配位双希夫碱萨尔芬共价有机框架(SCOF-Ru)的重新设计,作为半导体人工酶,具有放射性可激活的ros催化效率和抗肿瘤免疫,可抑制肿瘤转移和复发。实验和理论结果表明,半导体的SCOF-Ru具有较大的π共轭、高效的电子转移、强的电子空穴分离和独特的Ru2-N4O2催化中心,在低剂量x射线照射下具有最优越的ROS生成能力。而不是依赖于高z元素,具有优化能带结构的半导体SCOF-Ru赋予Ru位点高辐射敏化效应。我们的研究结果表明,SCOF-Ru不仅可以有效抑制DNA修复,还可以通过下调钙信号通路引发细胞凋亡。相应的,SCOF-Ru的治疗优势和抑制复发的效果已经在不同的肿瘤模型中得到了验证,特别是在放疗耐药的患者来源的异种移植模型中。结合免疫检查点阻断,放射性激活的SCOF-Ru显示出强大的抑制远处肿瘤生长的潜力。我们相信,ros催化和放射性激活人工酶的创新设计将为治疗恶性肿瘤提供一条有希望的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


