Selective Biomass Valorization in Neutral Electrolyte by Lowering the O2 Activation Energy via Tandem Catalysis
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
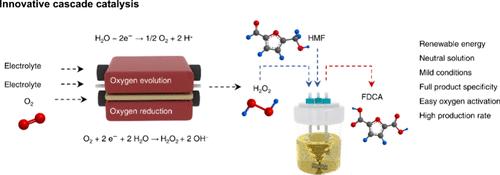
Conventional aerobic oxidation of biomass 5-hydroxymethylfurfural (HMF) encounters challenges due to the harsh conditions stemming from low solubility and high activation energy of O2, which hinder the efficient production of 2,5-furandicarboxylic acid (FDCA). Here, we propose a tandem catalytic system that lowers the activation energy for O2 to enable efficient and selective HMF oxidation to produce FDCA or 5-formyl-2-furoic acid (FFCA) under mild conditions. To overcome the high activation energy of O2 in the thermocatalytic processes, an electrochemical O2 activation process was employed to efficiently produce H2O2 as an oxidant to valorize HMF into either FDCA or FFCA. This tandem catalytic system achieved a cumulative FDCA yield of 2.21 mol gRu–1, full HMF conversion (100%), and complete product selectivity (100%), with an average productivity of 13.19 mmol gRu–1 h–1 over an extended 170 h operation period. The FDCA productivity in this work exceeds that in most aerobic oxidations under mild conditions. Further investigation into the detailed reaction process demonstrated that OH from H2O dissociation directly interacted with the aldehyde group in HMF to form a carboxylic acid group, while H2O2 serves as an oxidant to extract electrons from HMF or reaction intermediates. Furthermore, the efficient oxidation of structurally diverse substrates from EtOH to glycerol demonstrates the broad applicability of this tandem catalytic system.
通过串联催化降低O2活化能在中性电解质中选择性生物质增值
传统的生物质5-羟甲基糠醛(HMF)的好氧氧化工艺由于O2的低溶解度和高活化能等条件恶劣,阻碍了2,5-呋喃二羧酸(FDCA)的高效生产。在这里,我们提出了一个串联催化系统,降低O2的活化能,使HMF在温和的条件下有效和选择性地氧化生成FDCA或5-甲酰基-2-呋喃酸(FFCA)。为了克服热催化过程中O2的高活化能,采用电化学O2活化工艺高效地生成H2O2作为氧化剂,使HMF转化为FDCA或FFCA。该串联催化体系的FDCA累计产率为2.21 mol gRu-1, HMF转化率为100%,产物选择性为100%,在170 h的操作周期内平均产率为13.19 mmol gRu-1 h - 1。在这项工作中,FDCA的生产率超过了大多数温和条件下的有氧氧化。进一步详细的研究表明,H2O解离产生的OH直接与HMF中的醛基相互作用形成羧酸基,而H2O2作为氧化剂从HMF或反应中间体中提取电子。此外,从EtOH到甘油结构多样的底物的有效氧化表明了该串联催化系统的广泛适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


