Structural Variation and Charge-Transfer Dynamics of Protonated β-Ketoenamine-Linked Covalent Organic Framework for Boosted Photocatalytic H2 Evolution
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The surface characteristics of covalent organic framework (COF) photocatalysts can be intentionally altered by the protonated treatment, a straightforward postmodification process. This study provides some insights into the specific variation in crystallinity, microstructures, chemical bonds, charge-transfer dynamics mechanism, electronic distribution, and activation energy resulting from the introduction of protons in a β-ketoenamine-linked COF (TpPa-1). It demonstrates that the introduced protons are attached to the –NH of β-ketoenamine groups to form the –NH···H+ groups. Moreover, it is found that the protonated TpPa-1 treated with 0.5 M HOAc solution exhibits a photocatalytic hydrogen evolution rate of 0.238 mmol h–1 in 100 mL ascorbic acid solution at the stationary point with the photocatalyst concentration of 400 mg L–1 without any cocatalysts under visible-light irradiation, equivalent to the mass-normalized value of 5.95 mmol h–1 g–1, which is about 40 and 20 times higher than that of pristine TpPa-1 and TpPa-1 treated with HCl solution, respectively. The isotopic labeling measurement demonstrated that both H2O and ascorbic acid molecules served as the sources of evolved H2. Furthermore, the significantly improved photocatalytic hydrogen evolution performance could be associated with the introduced protons and the acid–base adducts presented in the sample of TpPa-1-HOAc, as demonstrated by X-ray absorption near-edge spectra (XANES), which contributed to the promoted charge separation efficiency as well as the longer lifetime of photoexcited electrons. Additionally, density functional theory (DFT) calculations reveal that the protonated TpPa-1-HOAc shows special electron density redistribution and favorable Gibbs free energy (ΔGH*), which contributed to the significantly boosted photocatalytic H2 evolution performance.
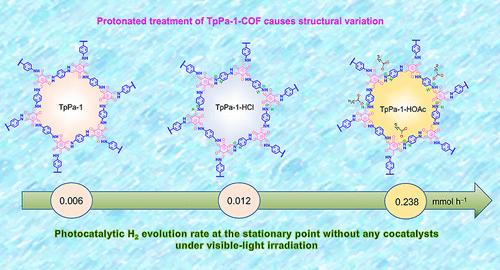
质子化β-酮胺键合共价有机骨架促进光催化H2演化的结构变化和电荷转移动力学
共价有机骨架(COF)光催化剂的表面特性可以通过质子化处理有意地改变,这是一种直接的后修饰过程。本研究对β-酮胺连接COF (TpPa-1)的结晶度、微观结构、化学键、电荷转移动力学机制、电子分布和活化能的具体变化提供了一些见解。结果表明,引入的质子与β-酮胺基的-NH相连,形成-NH···H+基团。此外,它是发现,质子化了的TpPa-1处理0.5霍阿克展品解决光催化氢进化速率为0.238 h -更易在抗坏血酸溶液100毫升驻点的光催化剂浓度400毫克l - 1:没有任何可见光辐照下,相当于5.95的mass-normalized价值更易与h - g - 1,级约40和20倍的原始TpPa-1 TpPa-1盐酸处理解决方案,分别。同位素标记表明H2O和抗坏血酸分子都是H2的来源。此外,x射线吸收近边光谱(XANES)表明,TpPa-1-HOAc样品中引入的质子和酸碱加合物可能与光催化析氢性能的显著提高有关,这有助于提高电荷分离效率和光激发电子的寿命。此外,密度泛函数理论(DFT)计算表明,质子化的TpPa-1-HOAc具有特殊的电子密度重分布和良好的吉布斯自由能(ΔGH*),这有助于显著提高光催化析氢性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


