Hydrophobic sponge spicule-mediated transdermal vaccination combines controlled skin inflammation as self-adjuvant with dual immune activation
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
We developed hydrophobic sponge (Haliclona sp.) spicules (hSHS) through surface modification of natural spicules (SHS) with monolayers of hydrophobic functional groups (e.g., alkyl and phenyl moieties). Utilizing ovalbumin (OVA) as a model protein antigen, we fabricated a transdermal vaccine (OVA@hSHS) through hydrophobic adsorption of OVA onto hSHS. Upon topical application, OVA@hSHS effectively penetrated the skin via microneedle-like mechanical puncture, enabling direct intradermal antigen access. Subsequent exposure to the physiological milieu triggered sustained antigen release with progressive diffusion into deeper skin layers. The system exhibited immunoadjuvant activity mediated by physical microinjury (i.e., skin cell disruption), which stimulated pro-inflammatory cytokine secretion and induced macrophage polarization toward the M1 phenotype. Within this inflammatory microenvironment, recruited antigen-presenting cells efficiently internalized and processed antigens, leading to enhanced maturation and antigen presentation. This ultimately elicited a robust and balanced immune response—combining potent humoral and cellular immunity with potential durable memory effects. Notably, the OVA@hSHS-induced skin inflammation was self-limiting, with skin tissue capable of autonomously restoring inflammatory homeostasis. In summary, hSHS offers a simple yet versatile platform for developing transdermal protein-based vaccines.
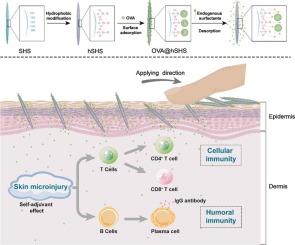
疏水海绵针状体介导的透皮疫苗结合了控制皮肤炎症作为自佐剂和双重免疫激活
利用疏水官能团(如烷基和苯基部分)对天然针状体(SHS)进行单层表面改性,制备了疏水海绵针状体(hSHS)。以卵清蛋白(OVA)为模型蛋白抗原,利用OVA在hSHS上的疏水吸附制备透皮疫苗(OVA@hSHS)。在局部应用后,OVA@hSHS通过微针状机械穿刺有效地穿透皮肤,使皮内抗原直接进入。随后暴露于生理环境中触发了持续的抗原释放,并逐渐扩散到更深的皮肤层。该系统表现出由物理微损伤(即皮肤细胞破坏)介导的免疫佐剂活性,刺激促炎细胞因子分泌,诱导巨噬细胞向M1表型极化。在这种炎症微环境中,募集的抗原提呈细胞有效地内化和加工抗原,导致成熟和抗原提呈增强。这最终引发了一种强大而平衡的免疫反应——结合了强大的体液和细胞免疫以及潜在的持久记忆效应。值得注意的是,OVA@hSHS-induced皮肤炎症是自限性的,皮肤组织能够自主恢复炎症稳态。总之,hSHS为开发透皮蛋白疫苗提供了一个简单而通用的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


