Pt–Co spatially separated catalyst for efficient and stable propane dehydrogenation reaction
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
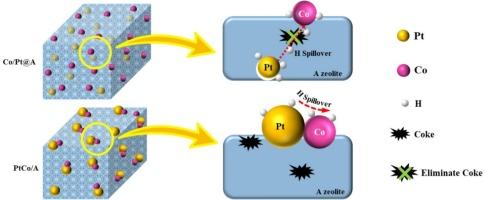
Propane dehydrogenation (PDH) is a promising method to satisfy the growing demand for propylene. Still, the traditional Pt-based catalysts are susceptible to sintering and coking, which leads to the deactivation of the catalysts; thus, developing stable and efficient catalysts for PDH is of significant importance to the industry. Herein, we prepared an efficient and stable PDH catalyst (Co/Pt@A) by encapsulating Pt particles in A zeolite and then loading Co species onto its surface. A co-impregnated PtCo/A catalyst was also prepared for comparison. The results show that the agglomeration and growth of Pt and Co particles of Co/Pt@A catalyst are effectively prevented during high-temperature reactions. Furthermore, the hydrogen spillover effect of the Co/Pt@A catalyst is significantly enhanced. The H species on Pt particles migrates through the A zeolite to the surface Co particles, enabling the coke precursors on the support to be quickly saturated and eliminated, reducing coke formation. In contrast, the Pt–Co closely contacted structure of the PtCo/A catalyst leads to the sintering of PtCo particles and a limited hydrogen spillover on the support surface. The catalytic performance in propane dehydrogenation shows that after 6 h, the C3H8 conversion and C3H6 selectivity of the Co/Pt@A catalyst are higher than those of the PtCo/A catalyst. This bimetallic spatially separated structure provides a new insight for the design and preparation of efficient catalysts for the PDH and many other reactions.
高效稳定丙烷脱氢反应的Pt-Co空间分离催化剂
丙烷脱氢(PDH)是满足日益增长的丙烯需求的一种很有前途的方法。然而,传统的pt基催化剂容易烧结和结焦,从而导致催化剂失活;因此,开发稳定高效的PDH催化剂具有重要的工业意义。在此,我们将Pt颗粒包埋在A沸石中,然后在其表面装载Co物质,制备了一种高效稳定的PDH催化剂(Co/Pt@A)。还制备了共浸渍PtCo/A催化剂进行比较。结果表明,在高温反应过程中,Co/Pt@A催化剂有效地阻止了Pt和Co颗粒的团聚和生长。Co/Pt@A催化剂的氢溢出效应显著增强。Pt颗粒上的H通过A沸石迁移到表面的Co颗粒上,使载体上的焦炭前驱体迅速饱和和消除,减少了焦炭的形成。相比之下,PtCo/A催化剂的Pt-Co紧密接触结构导致PtCo颗粒的烧结和有限的氢溢出在支撑表面。丙烷脱氢的催化性能表明,在6 h后,Co/Pt@A催化剂的C3H8转化率和C3H6选择性均高于PtCo/A催化剂。这种双金属空间分离结构为设计和制备PDH等反应的高效催化剂提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


