Novel furanone-based anticancer agents: Design, synthesis, Hsp90 inhibition, in vivo antitumor activity and pharmacokinetic studies
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
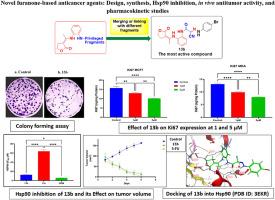
Novel furanone-based hybrids (series A-I) were designed and synthesized as potential anticancer agents and Hsp90 inhibitors using the privileged fragment combination strategy. NCI single-dose full panel assay identified 13b (series I) as the most active hit, demonstrating broad spectrum antiproliferative activity against 48 NCI cell lines (GI50 = 2.17 - 9.60 μM) and exhibiting total growth inhibition in 26 cell lines (TGI = 6.23 - 91.00 μM), with minimal toxicity toward 57 cell lines (LC50 > 92.5 μM) as revealed in the five-dose assay. 13b exerted strong cytotoxicity against MCF-7 and MDA-MB-231 cancer cells, with high selectivity over normal human skin fibroblast cells, according to the MTT assay. Mechanistic studies indicated that apoptosis was not the primary mechanism of activity. 13b suppressed proliferation by reducing colony formation and downregulating the proliferation marker Ki67. Additionally, it inhibited Hsp90, suppressed Hsp90 ATPase inhibition activity, downregulated client oncoproteins HER2 and CDK4, and induced a moderate Heat Shock Response. In a TNBC xenograft model, 13b significantly reduced tumor volume and weight with a better safety profile than 5-fluorouracil. Besides, 13b significantly suppressed EGFR expression as confirmed by immunohistochemistry analysis. In vivo pharmacokinetic studies revealed rapid systemic absorption (Cmax = 6.74 μM, Tmax = 1 h) and short half-life (T1/2 = 2.98 h). Molecular docking studies showed that 13b binds within the Hsp90 ATP-binding pocket in a comparable pattern to the known inhibitors, SNX-2112 and GDM. These findings support 13b as a promising, well-tolerated Hsp90-targeted anticancer lead, especially for the treatment of breast cancer.
新型呋喃酮类抗癌药物:设计、合成、Hsp90抑制、体内抗肿瘤活性和药代动力学研究
利用特权片段组合策略,设计并合成了新型呋喃酮基杂合体(系列A-I),作为潜在的抗癌药物和Hsp90抑制剂。NCI单剂量全面板试验发现13b(系列I)是最活跃的攻击,对48个NCI细胞系(GI50 = 2.17 - 9.60 μM)显示出广谱的抗增殖活性,对26个细胞系(TGI = 6.23 - 91.00 μM)显示出总体生长抑制,对57个细胞系(LC50 > 92.5 μM)的毒性最小,在五剂量试验中显示。根据MTT试验,13b对MCF-7和MDA-MB-231癌细胞具有很强的细胞毒性,比正常的人皮肤成纤维细胞具有高选择性。机制研究表明,细胞凋亡不是活性的主要机制。13b通过减少菌落形成和下调增殖标记Ki67抑制增殖。此外,它还能抑制Hsp90,抑制Hsp90 ATPase抑制活性,下调客户癌蛋白HER2和CDK4,诱导中度热休克反应。在TNBC异种移植模型中,13b显著减少肿瘤体积和重量,安全性优于5-氟尿嘧啶。免疫组化分析证实,13b显著抑制EGFR的表达。体内药代动力学研究显示,全身吸收快(Cmax = 6.74 μM, Tmax = 1 h),半衰期短(T1/2 = 2.98 h)。分子对接研究表明,13b以与已知抑制剂SNX-2112和GDM相似的模式结合在Hsp90 atp结合口袋内。这些发现支持13b作为一种有希望的、耐受性良好的hsp90靶向抗癌先导物,特别是用于治疗乳腺癌。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


