Discovery of a Natural Small-Molecule Inhibitor to Novel Target STAT5A for Ischemic Stroke Therapy
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Signal transducer and activator of transcription 5A (STAT5A) is known to regulate the processes of various cancers but remains unexplored in neuroprotective effects, especially in ischemic stroke (IS). Based on an HPLC-DAD-HRESIMS-oriented strategy, 16 stilbenoid dimers [(+)-1/(−)-1 – (+)-9/(−)-9] were isolated from Heterosmilax yunnanensis (Liliaceae). Pharmacological evaluations showed that (±)-2 could remarkably alleviate neuronal ischemic damage in MCAO models. A four-step synthesis of (±)-2 was achieved in a stereo- and regioselective manner by a protecting-group-free strategy. The SAR analysis indicated the necessity of two hydroxy groups at C-3/C-4 or C-3/C-5 in the A ring. Using a chemoproteomics technology, STAT5A was identified as the direct cellular target of (±)-2. Mechanistically, (±)-2 conjugates with STAT5A by the key Lys644 residue to inhibit its phosphorylation, thus exerting anti-IS effects. Our findings demonstrate that STAT5A might be a novel target for IS therapy, and (±)-2 was the first natural anti-IS drug candidate targeting STAT5A.
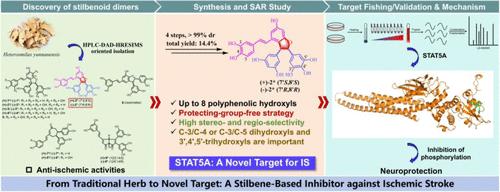
缺血性卒中治疗新靶点STAT5A的天然小分子抑制剂的发现
已知信号换能器和转录激活因子5A (STAT5A)调节各种癌症的过程,但尚未探索其神经保护作用,特别是在缺血性中风(is)中的作用。采用hplc - dad - hresms技术,从云南百合科杂木中分离得到16个二苯乙烯二聚体[(+)-1/(−)-1 -(+)-9/(−)-9]。药理评价显示(±)-2能显著减轻MCAO模型神经元缺血性损伤。通过无保护基团的策略,以立体和区域选择性的方式实现了(±)-2的四步合成。SAR分析表明,在A环的C-3/C-4或C-3/C-5上必须有两个羟基。利用化学蛋白质组学技术,STAT5A被鉴定为(±)-2的直接细胞靶点。机制上,(±)-2通过关键的Lys644残基与STAT5A结合,抑制其磷酸化,从而发挥抗is作用。我们的研究结果表明STAT5A可能是IS治疗的新靶点,而(±)-2是第一个针对STAT5A的天然抗IS候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


