Cell-free production of target-directed liposomes utilizing membrane localization peptides
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cell-free protein synthesis technology is a powerful tool for the rapid production of bioactive molecules such as antibodies. By combining with liposomes, immobilizing the synthesized proteins onto the liposome membrane leads production of bioactive liposomes within the cell-free reaction. These cell-free approaches can be applied in medical treatment and drug development, however, not many studies have been done to develop it as a highly versatile platform. Here, we explored hydrophobic amino acid sequences that lead hydrophilic proteins onto the liposome membrane and applied them to the co-translational production of bioactive liposomes. The membrane localizing peptides (MLPs) introduced at the N- or C-terminus of small molecular weight antibodies, nanobody, guided the membrane localization onto liposomes. The nanobodies-decorated liposomes specifically bound to cancer cells expressing the target antigen protein, thus suggesting maintaining functional structure on the liposome membrane. The MLPs we discovered can be widely applied to the rapid immobilization of proteins on liposome surfaces, leading to cell-free applications in virus-like particles or RNA therapy.
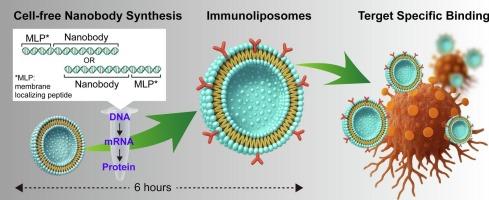
利用膜定位肽无细胞生产靶向脂质体
无细胞蛋白合成技术是快速生产抗体等生物活性分子的有力工具。通过与脂质体结合,将合成的蛋白质固定在脂质体膜上,在无细胞反应中产生生物活性脂质体。这些无细胞方法可以应用于医学治疗和药物开发,但是,将其开发为一个高度通用的平台的研究并不多。在这里,我们探索了将亲水性蛋白引入脂质体膜的疏水氨基酸序列,并将其应用于生物活性脂质体的共翻译生产。膜定位肽(MLPs)在小分子量抗体纳米体的N端或c端引入,引导膜定位到脂质体上。纳米体修饰的脂质体特异性地与表达靶抗原蛋白的癌细胞结合,从而表明脂质体膜上保持了功能结构。我们发现的mlp可以广泛应用于脂质体表面蛋白质的快速固定化,从而在病毒样颗粒或RNA治疗中实现无细胞应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


