Boron-Doped Carbon Material Modulated Highly Reactive Near-Free Fe(III) for Accelerated and Long-Lasting Fenton Oxidation with Hydrogen Peroxide as a Green Electron Donor
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Heteroatom doping has been applied as an excellent method for mediating the catalytic reactivity of carbon materials. To overcome the inherent defect of slow Fe(III) reduction in Fenton chemistry, this work finds that boron-doped carbon materials demonstrate high cocatalytic activity in boosting Fenton oxidation. During Fenton chain reactions catalyzed by boron-doped reduced graphene oxide (B-rGO, the typical boron-doped carbon material), highly reactive near-free Fe(III) formed on the B-rGO surface can dramatically promote H2O2 mediated Fe(III) reduction, which strongly accelerates the rate-determining reaction in Fenton chain reactions to generate hydroxyl radicals. The results of DFT calculations, electrochemical analysis, characterizations (XAFS, in situ Raman, and HAADF-STEM), and pH-dependent performance reveal that doped boron atoms can modulate the electron-deficient iron atom of FeOH2+ by stretching the Fe–O bond (from 163.6 to 179.2 pm), which forms near-free Fe(III) on the B-rGO surface with higher oxidation capability than free Fe(III), and the Fe(III) oxidation potential is positively and linearly related to the boron content of B-rGO (R2 = 0.96). Moreover, near-free Fe(III) can dramatically oxidize H2O2 (a green electron donor) to accelerate Fe(II) regeneration, thereby promoting Fenton chain reactions for long-lasting Fenton oxidation. These discoveries afford a strategy for designing green and sustainable cocatalysts to overcome the intrinsic weaknesses of classical Fenton chemistry.
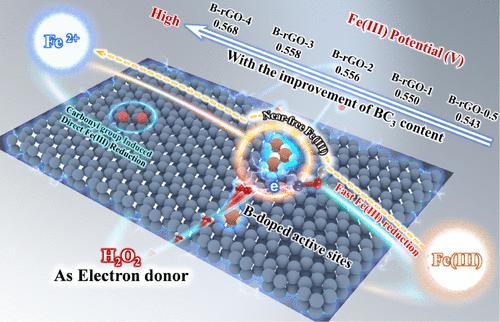
硼掺杂碳材料调制高活性近游离Fe(III)在过氧化氢加速和持久芬顿氧化中作为绿色电子供体
杂原子掺杂作为一种优良的碳材料催化活性中介方法已被广泛应用。为了克服Fenton化学中Fe(III)还原缓慢的固有缺陷,本工作发现硼掺杂碳材料在促进Fenton氧化方面表现出较高的共催化活性。在硼掺杂还原性氧化石墨烯(B-rGO,典型的硼掺杂碳材料)催化的Fenton链式反应中,在B-rGO表面形成的高活性近游离Fe(III)可以显著促进H2O2介导的Fe(III)还原,从而强烈加速Fenton链式反应中的定速反应生成羟基自由基。DFT计算、电化学分析、表征(XAFS、原位拉曼和HAADF-STEM)和ph依赖性能的结果表明,掺杂硼原子可以通过拉伸Fe - o键(从163.6 pm到179.2 pm)来调节缺电子的FeOH2+铁原子,在B-rGO表面形成近自由的Fe(III),具有比自由Fe(III)更高的氧化能力。Fe(III)氧化电位与B-rGO中硼含量呈线性正相关(R2 = 0.96)。此外,近乎游离的Fe(III)可以显著氧化H2O2(绿色电子供体),加速Fe(II)的再生,从而促进Fenton链反应,实现持久的Fenton氧化。这些发现为设计绿色和可持续的助催化剂提供了一种策略,以克服经典芬顿化学的内在弱点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


