Discovery of Metabolic Cross-Coupling in Phenol-Arylamine Mixtures by Cytochrome P450 via Combined Computational and Experimental Approaches
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
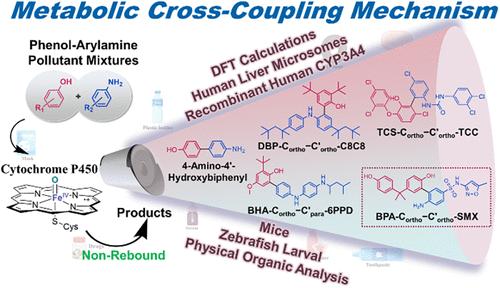
This study unveils a novel cytochrome P450 (P450)-mediated metabolic pathway that drives cross-coupling between diverse phenol and arylamine pollutants. Phenols and arylamines constitute a large part of industrial chemicals, pharmaceuticals, and personal care products, yet the biotransformation of phenol-arylamine pollutant mixtures remains largely unexplored. Density functional theory calculations revealed that the rate-limiting rebound barriers of phenoxy and arylamino radicals formed through O–H/N–H abstraction by the P450 catalytic oxidant, Compound I, facilitate their dissociation from the heme, creating thermodynamically favorable conditions for subsequent nonrebound cross-coupling reactions. These proposed hybrid products were systematically identified and quantified using various mass spectrometry technologies across multiple biological systems, including human liver microsomes, recombinant human CYP3A4, mice, and zebrafish. From a physical organic chemistry perspective, the widespread occurrence of cross-coupling is driven by sufficient lifetime of phenoxy and arylamino radicals due to spin delocalization, and their concentration gradient sustained by persistent radical effects. Notably, yeast two-hybrid assays demonstrated that the phenol-arylamine hybrids exhibited supra-additive estrogenic activity; for instance, the bisphenol A–sulfamethoxazole dimer with a hydrolytic half-life of 11.6 days displayed approximately 130-fold higher estrogenic activity than that of bisphenol A. The mechanism’s prevalence suggests an unrecognized bioactive pathway in chemical cocktails, thereby necessitating consideration of metabolism-driven reactive interactions in mixtures.
通过计算和实验相结合的方法发现细胞色素P450在苯酚-芳胺混合物中的代谢交叉偶联
本研究揭示了一种新的细胞色素P450 (P450)介导的代谢途径,该途径驱动多种苯酚和芳胺污染物之间的交叉偶联。苯酚和芳胺构成了工业化学品、药品和个人护理产品的很大一部分,但苯酚-芳胺污染物混合物的生物转化仍在很大程度上未被探索。密度泛函理论计算表明,P450催化氧化剂化合物I通过O-H / N-H萃取形成的苯氧基和芳基自由基的限速反弹屏障促进了它们与血红素的分离,为随后的非反弹交叉偶联反应创造了热力学有利条件。这些提议的杂交产物通过多种生物系统,包括人肝微粒体、重组人CYP3A4、小鼠和斑马鱼,使用各种质谱技术进行系统鉴定和定量。从物理有机化学的角度来看,交叉偶联的广泛发生是由于自旋离域导致的苯氧基和芳基自由基具有足够的寿命,以及持续的自由基效应维持了它们的浓度梯度。值得注意的是,酵母双杂交实验表明,酚-芳胺杂交表现出超加性雌激素活性;例如,水解半衰期为11.6天的双酚a -磺胺甲新唑二聚体显示出比双酚a高约130倍的雌激素活性。这种机制的普遍存在表明,在化学混合物中存在一种未被识别的生物活性途径,因此有必要考虑混合物中代谢驱动的反应相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


