CEP76 impairment at the centrosome-cilium interface contributes to a spectrum of ciliopathies
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Dysfunction at the centrosome-cilium interface underlies a broad range of ciliopathies. Here, we identify biallelic variants in CEP76, encoding a centrosomal protein, in eight unrelated individuals presenting with neurodevelopmental, ocular, and variable additional multisystem features. Proband-derived fibroblasts and CEP76-depleted RPE1 cells display ciliary deficits, including impaired cilium formation and length, disrupted transition zone architecture, and impaired IFT88-mediated anterograde intraflagellar transport. Zebrafish cep76 mutants recapitulate key clinical phenotypes, and in vitro complementation assays confirm pathogenicity for all tested human disease-associated variants. Proteomics analysis identifies CEP76 interactors, including known partners CCP110 and CEP97, and highlights clinically and functionally relevant candidates, including ALMS1 and LUZP1. Together, these findings expand the role of CEP76 beyond centriole duplication to include ciliary assembly and trafficking, establishing it as a ciliopathy gene. This work provides mechanistic insights into CEP76-related disease and broadens our understanding of centrosome-cilium biology.
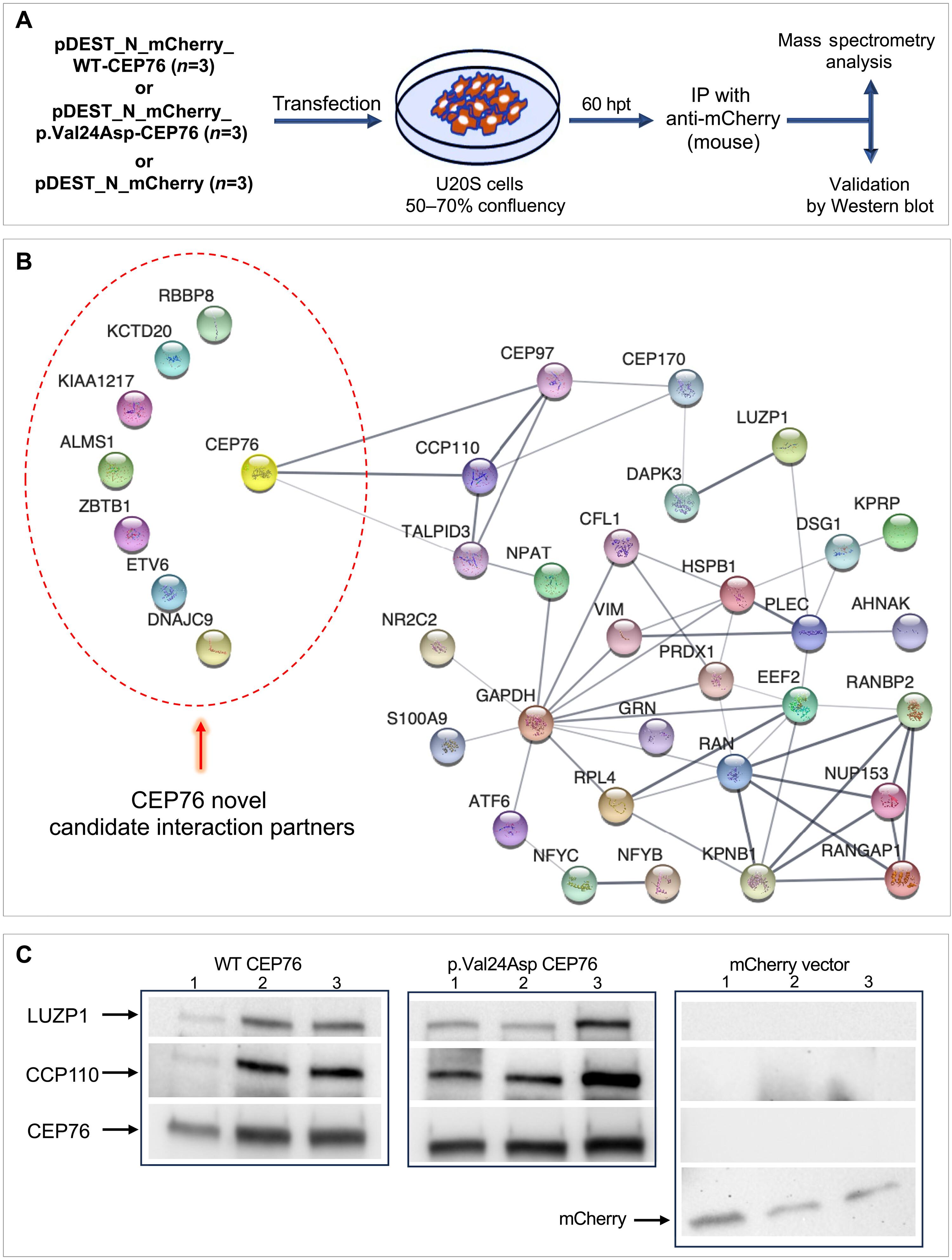
中心体-纤毛界面的CEP76损伤导致一系列纤毛病
中心体-纤毛界面的功能障碍是多种纤毛病的基础。在这里,我们在8个没有关系的个体中发现了编码中心体蛋白的CEP76双等位变异,这些个体表现出神经发育、眼部和可变的其他多系统特征。原源性成纤维细胞和cep76缺失的RPE1细胞表现出纤毛缺陷,包括纤毛形成和长度受损,过渡区结构破坏,ift88介导的纤毛内顺行运输受损。斑马鱼cep76突变体概括了关键的临床表型,体外互补试验证实了所有测试的人类疾病相关变异的致病性。蛋白质组学分析确定了CEP76相互作用因子,包括已知的合作伙伴CCP110和CEP97,并突出了临床和功能相关的候选因子,包括ALMS1和LUZP1。总之,这些发现将CEP76的作用扩展到中心粒复制之外,包括纤毛组装和运输,从而确定它是一种纤毛病基因。这项工作提供了CEP76相关疾病的机制见解,拓宽了我们对着丝体-纤毛生物学的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


