Kinesin-1 coordinates cross-talk between microtubule and actin cytoskeletons during dendritic cell migration
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Dendritic cells (DCs) are professional antigen (Ag)–presenting cells that excel in initiating adaptive immune responses by continuously scanning peripheral tissues for Ags. To facilitate efficient DC migration, constant cross-talk between actin and microtubules is required to coordinate cytoskeletal networks and actomyosin contractility, but the related mechanisms have not been extensively characterized. We show that mouse DCs lacking Kif5b (the heavy chain of kinesin-1) exhibit a major impairment in cell migration in vivo and in vitro. Mechanistically, kinesin-1 coordinates cytoskeletal cross-talk between actin and microtubules during DC migration by modulating negatively RhoA activity through its interaction with GEF-H1, thereby limiting GEF-H1’s availability in the cytosol. The same mechanism operates in human primary monocyte–derived DCs and regulates efficient migration in a confined environment. Thus, our results highlight kinesin-1 as a key regulator of DC migration, through its coordinated control of cytoskeletal dynamics.
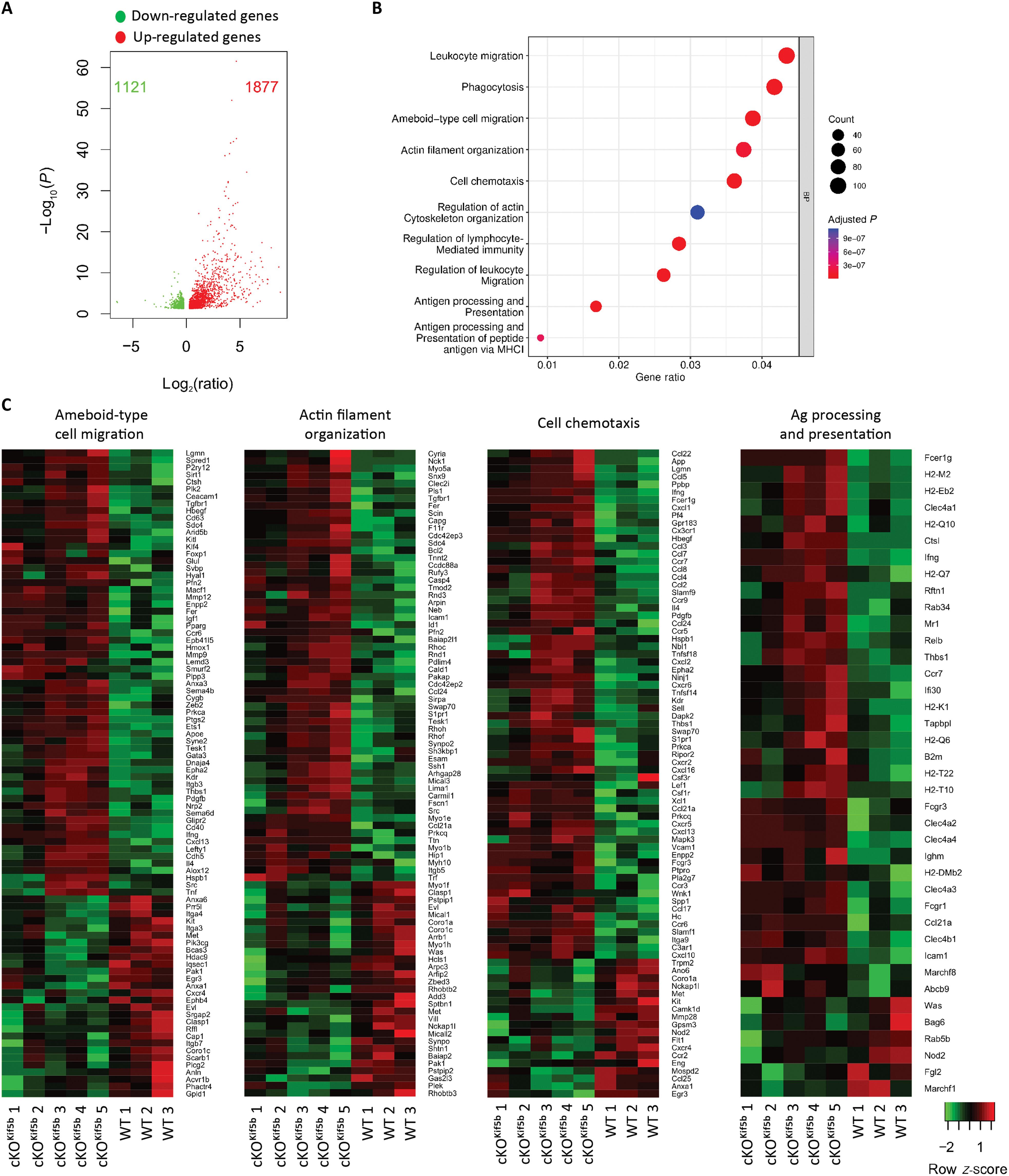
在树突状细胞迁移过程中,驱动蛋白-1协调微管和肌动蛋白细胞骨架之间的交叉对话
树突状细胞(dc)是一种专业抗原(Ag)呈递细胞,通过连续扫描外周组织寻找Ag,擅长启动适应性免疫反应。为了促进DC的有效迁移,肌动蛋白和微管之间需要持续的串扰来协调细胞骨架网络和肌动球蛋白的收缩性,但相关机制尚未得到广泛的表征。我们发现,缺乏Kif5b(运动蛋白-1的重链)的小鼠dc在体内和体外的细胞迁移中表现出严重的损伤。从机制上讲,在DC迁移过程中,激酶1通过与GEF-H1的相互作用调节负RhoA活性,从而限制GEF-H1在细胞质中的可用性,从而协调肌动蛋白和微管之间的细胞骨架串扰。同样的机制在人类原代单核细胞衍生的dc中起作用,并调节在受限环境中的有效迁移。因此,我们的研究结果强调了激酶-1作为DC迁移的关键调节因子,通过其协调控制细胞骨架动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


