Decoupling charge and ion transport in oxygen evolution reaction through surface hydration engineering of molecular graphene catalysts
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Interfacial microenvironments play a critical role in regulating the kinetics and stability of the oxygen evolution reaction (OER), yet their dynamic effects remain poorly understood. Herein, we developed a molecularly defined catalyst platform to systematically investigate the influence of surface hydration layers on OER activity. A hydrazinecarbonylferrocene-functionalized nitrogen-doped graphene (HFc-NG) electrocatalyst was synthesized and subsequently coated with a polyvinyl alcohol (PVA)-derived hydrogel layer to form HFc-NG-OH, enabling a direct comparison between hydrated and non-hydrated interfaces. Electrochemical measurements revealed that HFc-NG-OH exhibits significantly enhanced OER performance, characterized by lower overpotentials, reduced Tafel slopes, and improved long-term stability under both alkaline and neutral conditions. Redox probe analysis and electrochemical impedance spectroscopy indicated that the hydration layer facilitates inner-sphere electron transfer and ion transport through hydrogen-bonded networks. In situ ATR-FTIR spectroscopy further confirmed accelerated formation and stabilization of key OER intermediates at the hydrated interface. This work underscores the dual functionality of hydration layers in modulating interfacial charge dynamics and intermediate evolution, offering valuable insights into surface hydration engineering for advanced electrocatalysis.
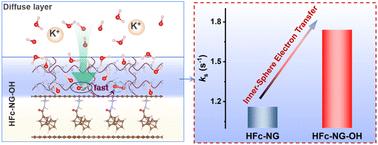
分子石墨烯催化剂表面水化工程解耦析氧反应中的电荷和离子输运
界面微环境对析氧反应(OER)的动力学和稳定性起着关键的调节作用,但其动力学效应尚不清楚。在此,我们开发了一个分子定义的催化剂平台来系统地研究表面水化层对OER活性的影响。合成了一种肼羰基二茂铁功能化氮掺杂石墨烯(HFc-NG)电催化剂,并在其表面涂覆聚乙烯醇(PVA)衍生的水凝胶层,形成HFc-NG- oh,从而可以直接比较水合界面和非水合界面。电化学测量结果表明,HFc-NG-OH在碱性和中性条件下均表现出较低的过电位、较低的Tafel斜率和较好的长期稳定性,显著提高了OER性能。氧化还原探针分析和电化学阻抗谱分析表明,水化层促进了球内电子传递和离子通过氢键网络传递。原位ATR-FTIR光谱进一步证实了水合界面处关键OER中间体的加速形成和稳定。这项工作强调了水合层在调节界面电荷动力学和中间演化方面的双重功能,为高级电催化的表面水合工程提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


