Simultaneous induction of multiple classes of broadly neutralizing antibody precursors by combination germline-targeting immunization in nonhuman primates
IF 16.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
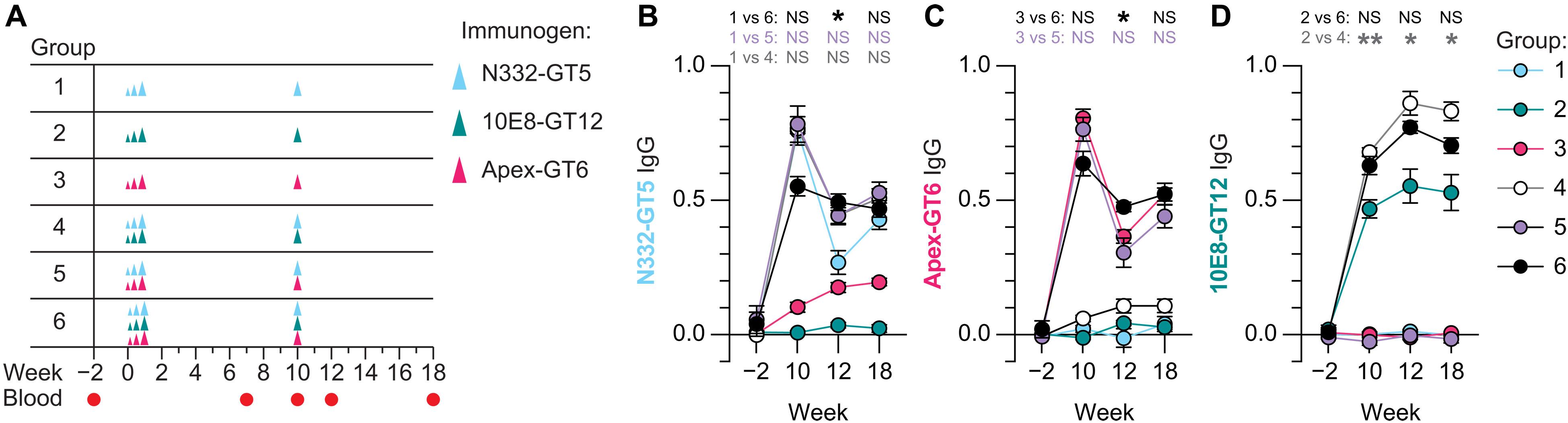
Inducing broadly neutralizing antibodies (bnAbs) against HIV remains a key challenge in vaccine development. Germline-targeting immunogens have effectively primed bnAb B cell lineages to individual HIV envelope epitopes in humans and nonhuman primates. However, eliciting consistent bnAb breadth requires the induction of multiple bnAb classes. We investigated whether immunization with a combination of germline-targeting immunogens could concurrently prime multiple bnAb lineages in nonhuman primates. Animals were immunized with three immunogens, targeting distinct epitopes: the V3-glycan/N332 supersite, the V2 Apex region, and the membrane-proximal external region (MPER), either individually or in combinations of two or all three. Triple combination immunization transiently reduced V2 Apex and V3-glycan responses, but by 8 weeks postboost, bnAb precursor lineages were observed to all three epitopes. Similar somatic hypermutation was observed across groups, indicative of permissive germinal center responses. These findings support combination germline-targeting immunization as a viable strategy to prime multiple bnAb lineages simultaneously.
在非人灵长类动物中通过结合种系靶向免疫同时诱导多种广泛中和的抗体前体
诱导抗艾滋病毒的广泛中和抗体(bnAbs)仍然是疫苗开发中的一个关键挑战。生殖系靶向免疫原已在人类和非人灵长类动物中有效地将bnAb细胞系引至单个HIV包膜表位。然而,诱导一致的bnAb宽度需要诱导多个bnAb类别。我们研究了用生殖系靶向免疫原组合免疫是否可以同时在非人灵长类动物中启动多个bnAb谱系。用三种免疫原免疫动物,分别针对不同的表位:v3 -聚糖/N332超位、V2顶点区和膜近端外区(MPER),可以单独免疫,也可以联合免疫三种免疫原或全部免疫原。三联免疫暂时降低了V2顶点和v3聚糖反应,但在增强后8周,在所有三个表位上都观察到bnAb前体谱系。各组间观察到相似的体细胞超突变,表明生发中心的宽容反应。这些发现支持结合种系靶向免疫作为一种可行的策略,同时启动多个bnAb谱系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


