Bacterial communities co-develop with respiratory immunity early in life, linking dysbiosis to systemic monocyte signature and wheezing
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
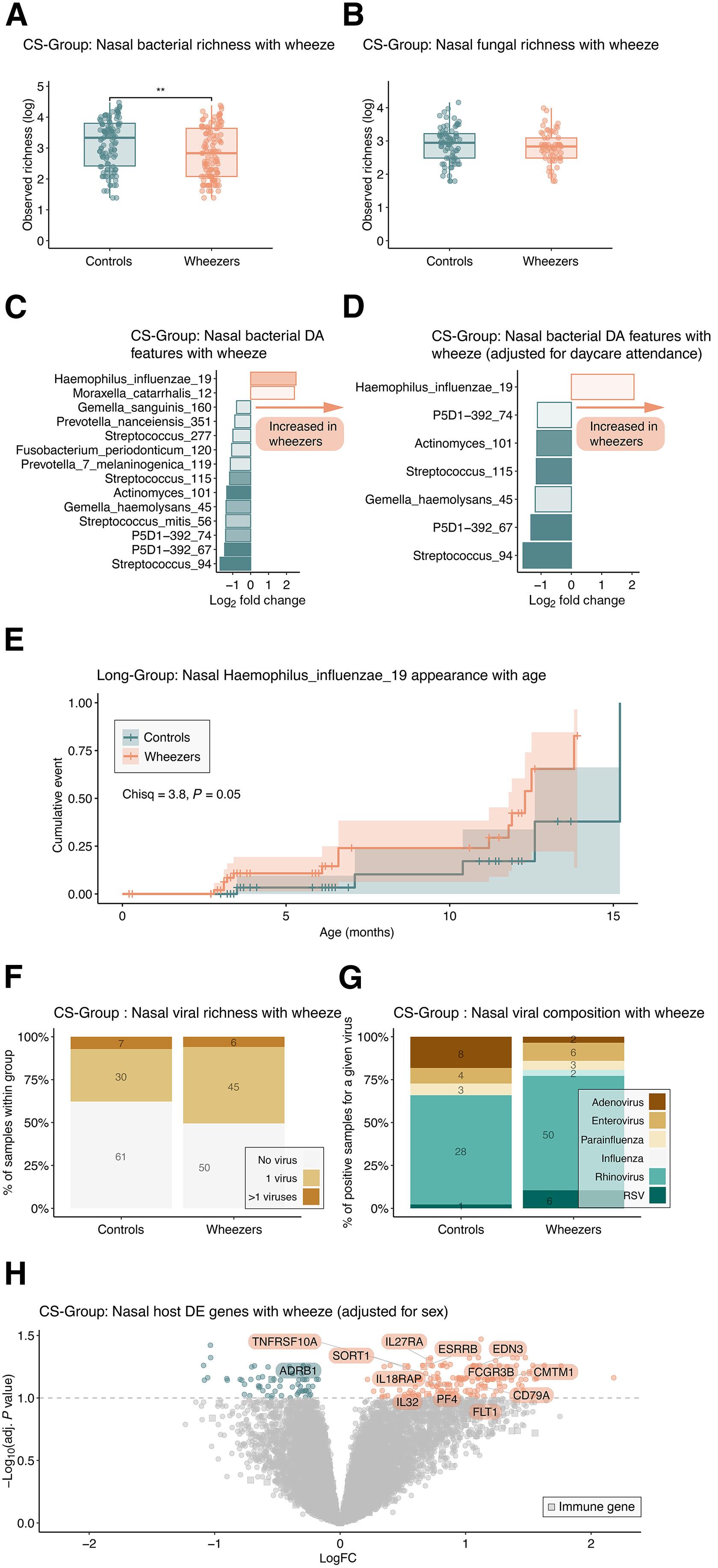
Early microbial colonization influences respiratory disease risk, yet mechanisms remain unclear. In a prospective birth cohort of 256 infants, we profiled bacterial, fungal, and viral communities in the upper airway and assessed local immune gene expression longitudinally and systemic gene expression at 1 year. Bacterial populations, not fungal or viral, correlated most strongly with immune development during the first 3 months, coinciding with composition shifts and immune-related gene expression changes, including interferon and adaptive immunity pathways. In contrast, the mycobiome and resident viruses showed no significant coevolution with host immunity. By 1 year, infants who previously wheezed displayed an upper airway microbiota enriched in Haemophilus influenzae and Moraxella, accompanied by a distinct local and systemic immune gene signature featuring elevated classical monocyte-related genes. These findings reveal a specific link between early-life bacterial dysbiosis, monocyte-related immunity, and wheezing onset, suggesting potential targets for early intervention in respiratory disease.
细菌群落在生命早期与呼吸免疫共同发展,将生态失调与系统性单核细胞特征和喘息联系起来
早期微生物定植影响呼吸道疾病风险,但机制尚不清楚。在256名婴儿的前瞻性出生队列中,我们分析了上呼吸道的细菌、真菌和病毒群落,并纵向评估了局部免疫基因表达和1年后的全身基因表达。细菌种群,而不是真菌或病毒,在头3个月与免疫发育最密切相关,与组成变化和免疫相关基因表达变化相吻合,包括干扰素和适应性免疫途径。相比之下,真菌组和驻留病毒与宿主免疫没有显着的共同进化。到1岁时,先前喘息的婴儿表现出上呼吸道微生物群富含流感嗜血杆菌和莫拉菌,并伴有明显的局部和全身免疫基因特征,其特征是经典单核细胞相关基因升高。这些发现揭示了生命早期细菌生态失调、单核细胞相关免疫和喘息发作之间的特定联系,提示了呼吸系统疾病早期干预的潜在目标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


