Development of an Optimized Synthetic Process for Onradivir Featuring a “One-Pot” Miyaura–Suzuki Coupling Reaction
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
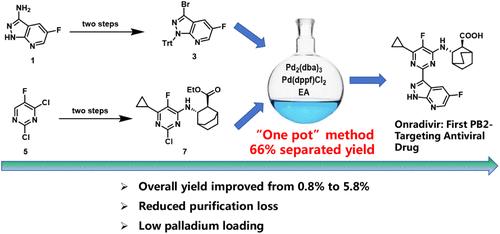
Influenza A virus (IAV) is a highly contagious pathogen responsible for significant global morbidity and mortality, with an estimated 1 billion infections annually. Onradivir, a next-generation PB2 inhibitor derived from Pimodivir, shows superior activity against drug-resistant IAV variants but faces manufacturing challenges. We report a scalable 7-step synthesis featuring two key innovations: (1) a silver-catalyzed radical cyclopropylation (89.5% conversion) replacing hazardous Grignard reagents; (2) a streamlined one-pot Miyaura–Suzuki coupling achieving 66% yield for intermediate 8. The route eliminates column chromatography through strategic recrystallizations, reduces Pd catalyst loading, and employs cost-effective ethyl acetate solvent. Process optimizations at 15 g scale demonstrate a consistent 5.8% yield for the API (representing a 7-fold improvement over the original method), with all intermediates either crystallized or telescoped to minimize purification losses. The developed methodology facilitates the commercial development of Onradivir and provides a general platform for the synthesis of structurally complex PB2 inhibitors.
以“一锅”miyura - suzuki偶联反应为特征的Onradivir合成优化工艺的开发
甲型流感病毒(IAV)是一种高度传染性病原体,造成全球大量发病率和死亡率,估计每年有10亿人感染。Onradivir是由Pimodivir衍生而来的下一代PB2抑制剂,对耐药IAV变体显示出卓越的活性,但面临生产挑战。我们报告了一种可扩展的7步合成方法,具有两个关键创新:(1)银催化的自由基环丙基化(转化率为89.5%)取代了危险的格氏试剂;(2)一种流线型的一锅miyura - suzuki联轴器,中间体产量达到66%。该方法通过战略性重结晶消除了柱层析,减少了钯催化剂的负载,并采用了经济高效的乙酸乙酯溶剂。在15g规模下的工艺优化表明,原料药的收率为5.8%(比原始方法提高了7倍),所有中间体要么结晶,要么伸缩,以最大限度地减少纯化损失。所开发的方法促进了Onradivir的商业开发,并为结构复杂的PB2抑制剂的合成提供了一个通用平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


