Photochemical Ni-Catalyzed Selective Mono-N-Arylation
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The selective synthesis of N-monomethyl arylamines presents an important challenge in synthetic chemistry. Furthermore, small molecule amines readily coordinate with metal catalysts, causing catalyst deactivation. In this work, we report an efficient C–N coupling reaction of (hetero)aryl halides with small molecule amine salts. Without the need for an external photosensitizer in the presence of a soluble organic base, a bipyridine-Ni(II) complex serving as a catalyst successfully enables the selective C–N coupling of (hetero)aryl halides with methylamine, deuterated methylamine, ethylamine, and dimethylamine under purple light irradiation (390–395 nm), thus preventing catalyst deactivation. Mechanistic studies reveal that the reaction proceeds via a Ni(I)–Ni(III) catalytic cycle, and more than 100 examples showcase its feasibility and versatility in organic synthesis.
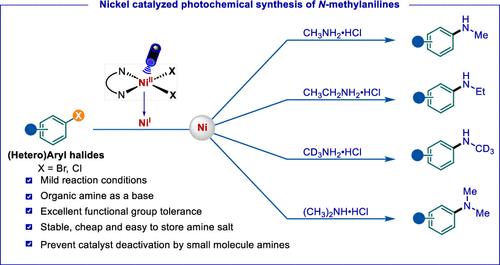
光化学镍催化选择性单- n -芳基化
n -单甲基芳胺的选择性合成是合成化学中的一个重要挑战。此外,小分子胺很容易与金属催化剂配合,导致催化剂失活。在这项工作中,我们报道了(杂)芳基卤化物与小分子胺盐的高效C-N偶联反应。在不需要外部光敏剂的情况下,在可溶性有机碱的存在下,联吡啶- ni (II)配合物作为催化剂,在紫外光照射(390-395 nm)下成功地实现了(杂)芳基卤化物与甲胺、氘化甲胺、乙胺和二甲胺的选择性C-N偶联,从而防止了催化剂失活。机理研究表明,该反应是通过Ni(I) -Ni (III)催化循环进行的,并且超过100个实例证明了其在有机合成中的可行性和通用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


