Secondary sulfidation-engineered Co/N–graphene catalyst for boosted ORR/OER bifunctionality in zinc–air batteries
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
The development of highly efficient and stable bifunctional catalysts is of crucial importance for advancing the large-scale application of zinc-air batteries (ZABs). This present study introduces a composite catalyst composed of cobalt-based sulfides supported on nitrogen-doped graphene (Co@NG-S2), synthesized through a two-step sulfurization strategy. The first sulfurization stage was successful in achieving co-doping of sulfur (S) and nitrogen (N) while enhancing the conductivity and activity of the graphene support. The second sulfurization step promoted the uniform distribution of cobalt-based sulfides and strengthened their interfacial coupling with graphene, thereby significantly improving their bifunctional catalytic performance. Electrochemical characterization revealed that Co@NG-S2 exhibited an oxygen reduction reaction (ORR) onset potential of 0.868 V and a half-wave potential (E1/2) of 0.798 V (vs. RHE). For the oxygen evolution reaction (OER), Co@NG-S2 demonstrated a low overpotential of merely 430 mV at a current density of 10 mA cm-2, thereby demonstrating exceptional ORR/OER bifunctional activity. In addition, a liquid ZAB assembled with this catalyst achieved a peak power density of 110 mW cm-2 and exhibited cycling stability exceeding 200 h. This study proposes a novel strategy for the synthesis of high-performance transition metal sulfide-carbon-based catalysts, demonstrating considerable promise for future applications.
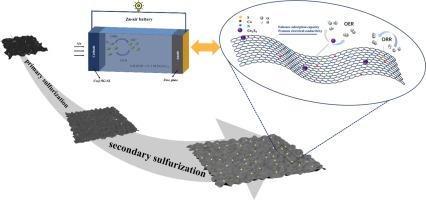
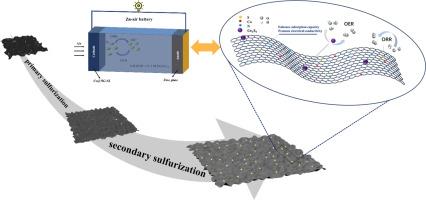
二次硫化工程Co/ n-石墨烯催化剂在锌-空气电池中提高ORR/OER双功能
开发高效、稳定的双功能催化剂对于推进锌空气电池(ZABs)的大规模应用至关重要。本研究介绍了一种由氮掺杂石墨烯(Co@NG-S2)支撑的钴基硫化物组成的复合催化剂,通过两步硫化策略合成。第一个硫化阶段成功地实现了硫(S)和氮(N)的共掺杂,同时提高了石墨烯载体的导电性和活性。第二硫化步骤促进了钴基硫化物的均匀分布,并加强了其与石墨烯的界面偶联,从而显著提高了其双功能催化性能。电化学表征表明Co@NG-S2的氧还原反应(ORR)起始电位为0.868 V,半波电位(E1/2)为0.798 V(相对于RHE)。对于析氧反应(OER), Co@NG-S2在电流密度为10 mA cm-2时显示出仅430 mV的低过电位,从而显示出特殊的ORR/OER双功能活性。此外,用该催化剂组装的液体ZAB达到了110 mW cm-2的峰值功率密度,并且具有超过200小时的循环稳定性。本研究提出了一种合成高性能过渡金属硫化物-碳基催化剂的新策略,具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


