Targeting PLOD2 induces epithelioid differentiation and improves therapeutic response in sarcomatoid renal cell carcinoma
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
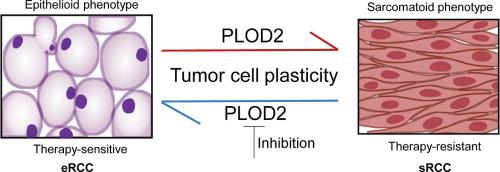
Sarcomatoid renal cell carcinoma (sRCC), a lethal variant arising through sarcomatoid dedifferentiation of epithelioid RCC (eRCC), poses significant clinical challenges due to the lack of molecular biomarkers and limited therapeutic options, with a median survival time for patients of less than 12 months.Objectives
To dissect the molecular basis of sarcomatoid dedifferentiation and develop novel therapeutic strategies for sRCC.Methods
Guided by the coprogenitor theory and tumor plasticity principles, we developed a differentiation-induction strategy targeting sarcomatoid dedifferentiation. Integrated transcriptomic and proteomic profiling revealed PLOD2 as a candidate therapeutic target. Spatial profiling of clinical sRCC specimens revealed selective PLOD2 overexpression in sarcomatoid components. Functional assays, including genetic ablation and pharmacological inhibition, were performed in sRCC cell lines and xenograft models to validate the role of PLOD2 in conferring sarcomatoid dedifferentiation plasticity and sarcomatoid morphology. Therapeutic significance was evaluated by PLOD2 intervention alone or in combination with conventional therapies (doxorubicin, gemcitabine, IFN-α, and axitinib).Results
Compared with neighboring epithelioid RCC and adjacent normal components, PLOD2 expression was markedly upregulated in sarcomatoid regions. PLOD2 fuelled sarcomatoid dedifferentiation plasticity by activating cancer stemness, dedifferentiation, and EMT, partially via downstream activation of DCLK1, a cancer stem cell marker. PLOD2 ablation reversed sarcomatoid phenotypes, restored epithelioid differentiation, and sensitized tumors to conventional RCC therapies. Notably, minoxidil, an FDA-approved PLOD2 inhibitor, effectively suppressed sarcomatoid features and synergized with standard treatments in preclinical models.Conclusion
Targeting PLOD2-mediated tumor plasticity represents a novel differentiation-inducing strategy to reprogram sRCC into therapy-responsive epithelioid states. The repurposing potential of minoxidil provides an immediate translatable strategy to improve clinical outcomes in this treatment-resistant malignancy.
靶向PLOD2诱导肉瘤样肾细胞癌的上皮样分化并提高治疗效果
肉瘤样肾细胞癌(sRCC)是一种由上皮样肾细胞癌(eRCC)的肉瘤样去分化引起的致命变异,由于缺乏分子生物标志物和有限的治疗选择,患者的中位生存时间不到12 个月,因此面临着重大的临床挑战。目的探讨肉瘤样细胞去分化的分子基础,探讨sRCC的新治疗策略。方法在共祖细胞理论和肿瘤可塑性原理的指导下,我们开发了一种针对类肉瘤去分化的分化诱导策略。综合转录组学和蛋白质组学分析显示PLOD2是候选的治疗靶点。临床sRCC标本的空间分析显示,PLOD2在肉瘤样成分中选择性过表达。在sRCC细胞系和异种移植模型中进行了功能分析,包括基因消融和药物抑制,以验证PLOD2在赋予肉瘤样去分化可塑性和肉瘤样形态方面的作用。通过PLOD2单独干预或联合常规治疗(阿霉素、吉西他滨、IFN-α和阿西替尼)评估治疗意义。结果与邻近上皮样RCC及邻近正常成分相比,肉瘤样区PLOD2表达明显上调。PLOD2通过激活癌症干细胞、去分化和EMT,部分通过下游的DCLK1(一种癌症干细胞标志物)激活,促进了类肉瘤的去分化可塑性。PLOD2消融逆转了肉瘤样表型,恢复了上皮样分化,并使肿瘤对常规RCC治疗敏感。值得注意的是,米诺地尔是一种fda批准的PLOD2抑制剂,在临床前模型中有效地抑制了肉瘤样特征,并与标准治疗协同。结论靶向plod2介导的肿瘤可塑性是一种新的分化诱导策略,可将sRCC重编程为治疗反应性上皮样状态。米诺地尔的重新利用潜力提供了一种即时可翻译的策略,以改善这种治疗抵抗性恶性肿瘤的临床结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


