CoFe alloy realizing enhanced Fe-bridged electron superhighways in Mott-Schottky heterojunctions for efficient water and urea electrolysis
IF 14.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
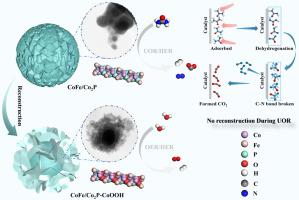
As a typical Mott-Schottky, the conventional single-metal/semiconductor junctions suffer from limited electronic modulation capability and reaction adaptability. This work developed a controllable synthesis strategy based on the Mott-Schottky theory and Prussian blue analog precursors, successfully constructing a CoFe/Co2P heterostructure by integrating bimetallic nanoalloys with phosphide semiconductors. Experimental characterization and theoretical calculations confirmed the continuous electron enrichment at the Co site. The low electronegativity of Fe, as the electron donor, expanded the interfacial work function difference (ΔΦ), and synergistically optimized the d-band electronic structure and intermediate adsorption by accelerating charge transfer kinetics. Consequently, the CoFe/Co2P heterostructure exhibits superior multi-reaction activity compared to pure Co2P and Co/Co2P in the urea oxidation reaction, oxygen evolution reaction (OER), and hydrogen evolution reaction in alkaline media. In situ characterization further demonstrated that the urea molecule was directly oxidized at the CoFe/Co2P interface site, avoiding the formation of the rate-limiting step CoOOH in the OER, which has rarely been mentioned in previous studies, thereby achieving faster kinetics at a significantly reduced potential. Accordingly, the assembled two-electrode urea oxidation-assisted hydrogen production electrolytic cell only requires 1.314 V to drive a current density of 10 mA cm−2, which is lower than the overall water splitting (1.691 V). This research provides a new interface control strategy for the rational design of efficient hydrogen production catalysts.
CoFe合金在Mott-Schottky异质结中实现了增强的铁桥电子高速公路,用于高效的水和尿素电解
传统的单金属/半导体结是典型的莫特-肖特基结,其电子调制能力和反应适应性有限。本工作基于Mott-Schottky理论和普鲁士蓝模拟前驱体开发了一种可控合成策略,通过将双金属纳米合金与磷化物半导体集成,成功构建了CoFe/Co2P异质结构。实验表征和理论计算证实了Co位的连续电子富集。Fe作为电子供体的低电负性扩大了界面功函数差(ΔΦ),并通过加速电荷转移动力学协同优化了d带电子结构和中间吸附。因此,在碱性介质中,CoFe/Co2P异质结构在尿素氧化反应、析氧反应(OER)和析氢反应中表现出比纯Co2P和Co/Co2P更高的多反应活性。原位表征进一步证明尿素分子在CoFe/Co2P界面位点被直接氧化,避免了在OER中形成限速步骤CoOOH,这在以往的研究中很少被提及,从而在显著降低的电位下实现了更快的动力学。因此,组装的双电极尿素氧化辅助制氢电解槽仅需1.314 V即可驱动10 mA cm−2的电流密度,低于整体水分解(1.691 V)。该研究为合理设计高效产氢催化剂提供了一种新的界面控制策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


