Microbial metabolism dysfunction induced by transarterial chemoembolization aggravates postprocedural liver injury in HCC
IF 33
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Transarterial chemoembolization (TACE) is widely used for treating unresectable hepatocellular carcinoma (HCC). Liver injury induced by TACE (TACE-LI) is the most common complication of TACE which limits long-term outcomes of HCC. Beyond traditional cognition of the direct damage induced by TACE on normal liver tissue, deeper mechanism underlying TACE-LI remains unclear.We aimed to further elucidate the unclear relationship between gut microbiota disturbances and TACE-LI.Methods
Microbial multi-omics analysis, genetically engineered bacteria and transcriptomics were used to study microbiota disturbances and host responses in TACE-LI.Results
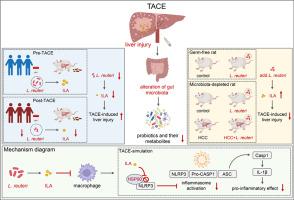
Rats with gut microbiota depleted by antibiotics and rats that received fecal transplants from donor rats or HCC patients that had undergone TACE showed more severe TACE-LI. Limosilactobacillus reuteri (L. reuteri) abundance was significantly reduced in TACE-treated rats and patients with HCC. Reduced L. reuteri abundance after TACE led to decreased levels of tryptophan metabolite indole-3-lactic acid (ILA), while administration of live L. reuteri or ILA provided effective protection against TACE-LI. Mechanistically, L. reuteri relied on the key enzyme phenyllactate dehydrogenase (fldH) to generate ILA, which inhibited the ATPase activity of heat shock protein 90 to deactivate NOD-like receptor protein 3-inflammasome in macrophages and suppressed hepatic pro-inflammatory response. Reduced levels of L. reuteri and ILA were correlated with aggravated LI and poor overall survival in TACE-treated patients with HCC.Conclusions
This is the first study to identify gut microbiota disturbance, i.e., deficiency of L. reuteri metabolite ILA, as significant cause of TACE-LI. L. reuteri and ILA administration serves as promising therapeutic approach for TACE-LI, which is crucial for reducing TACE adverse effects to achieve better prognosis in HCC.Impact and implications
The majority of patients with hepatocellular carcinoma (HCC) are diagnosed losing the chance of surgical resection. Transarterial chemoembolization (TACE) is widely used for treating unresectable HCC; however, its long-term outcome is significantly limited by its main complication, i.e., liver injury (LI). In addition to traditional cognition of the direct liver damage of the ischemic necrosis or regional chemotherapy induced by TACE, the deeper mechanisms underlying TACE-induced LI (TACE-LI) remain largely unclear. Gut microbiota can modulate various liver diseases but its exact role in TACE-LI has not been reported. We found that TACE could disturb the gut microbiota. This disturbance was characterized by reduced levels of Limosilactobacillus reuteri (L. reuteri) and its metabolite indole-3-lactic acid (ILA), which were correlated with aggravated TACE-LI and poor overall survival in HCC. Administration of L. reuteri or ILA significantly improved TACE-LI by inhibiting the inflammation of macrophages. Our study is the first report highlighting gut microbiota disturbances as an important cause of TACE-LI; administration of L. reuteri or ILA represents a viable and secure strategy for preventing TACE-LI, thereby reducing the adverse effects of TACE and yielding better prognoses in patients with HCC.
经动脉化疗栓塞引起的微生物代谢功能障碍加重肝细胞癌术后肝损伤
背景:经动脉化疗栓塞(TACE)被广泛用于治疗不可切除的肝细胞癌(HCC)。TACE诱导的肝损伤(TACE- li)是TACE最常见的并发症,它限制了HCC的长期预后。除了对TACE对正常肝组织直接损伤的传统认知外,TACE- li的深层机制尚不清楚。我们的目的是进一步阐明肠道微生物群紊乱与TACE-LI之间不明确的关系。方法采用微生物多组学分析、基因工程细菌和转录组学研究TACE-LI微生物群干扰和宿主反应。结果肠道菌群被抗生素耗尽的大鼠、接受供体大鼠粪便移植的大鼠或接受TACE的HCC患者的大鼠表现出更严重的TACE- li。在tace治疗的大鼠和HCC患者中,罗伊氏乳杆菌(L. reuteri)丰度显著降低。TACE后罗伊氏乳杆菌丰度降低导致色氨酸代谢物吲哚-3-乳酸(ILA)水平下降,而活的罗伊氏乳杆菌或ILA对TACE- li具有有效的保护作用。机制上,罗伊氏乳杆菌依靠关键酶苯乳酸脱氢酶(fldH)产生ILA,抑制热休克蛋白90 atp酶活性,使巨噬细胞内nod样受体蛋白3-炎性体失活,抑制肝脏促炎反应。在接受tace治疗的HCC患者中,罗伊氏乳杆菌和ILA水平降低与LI加重和总生存期差相关。结论本研究首次确定肠道菌群紊乱,即罗伊氏乳杆菌代谢物ILA缺乏是TACE-LI的重要原因。罗伊氏乳杆菌联合ILA治疗TACE- li是一种很有前景的治疗方法,对于减少TACE的不良反应以达到更好的HCC预后至关重要。影响和意义大多数肝细胞癌(HCC)患者被诊断为失去手术切除的机会。经动脉化疗栓塞(TACE)被广泛用于治疗不可切除的HCC;然而,其主要并发症肝损伤(LI)严重限制了其长期预后。除了对TACE引起的缺血性坏死或局部化疗的直接肝损伤的传统认知外,TACE诱导LI (TACE-LI)的更深层次机制仍不清楚。肠道微生物群可以调节多种肝脏疾病,但其在TACE-LI中的确切作用尚未报道。我们发现TACE会扰乱肠道菌群。这种紊乱的特征是罗伊氏乳杆菌(L. reuteri)及其代谢物吲哚-3-乳酸(ILA)水平降低,这与HCC中TACE-LI加重和总生存期差有关。罗伊氏乳杆菌或ILA通过抑制巨噬细胞的炎症显著改善TACE-LI。我们的研究是第一篇强调肠道微生物群紊乱是TACE-LI的重要原因的报告;给予罗伊氏乳杆菌或ILA是预防TACE- li的一种可行和安全的策略,从而减少TACE的不良反应,并使HCC患者预后更好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


