Switching the Oxygen Evolution Reaction Mechanism through the Creation of Disordered NiOOH Induced by Electrochemical Reconstruction
IF 26.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electrochemical reconstruction behavior of electrocatalysts during the oxygen evolution reaction (OER) is the key to determining their performance. Despite its critical role, precisely controlling and rationally guiding this reconstruction behavior remains an elusive challenge. Here, an efficient strategy is reported to manipulate the reconstruction behavior of nickel oxides by concurrently introducing amorphous structure and easily oxidizable elements (i.e., Mo6+). Specifically, the amorphous structure promotes the reconstruction at a low potential and the oxidative removal of Mo6+, enabling the generation of disordered NiOOH (d-NiOOH) with abundant defects. Notably, the d-NiOOH markedly enhances the Ni–O covalency and thus triggers the reaction mechanism transition from the adsorption evolution mechanism (AEM) to the lattice oxygen-mediated mechanism (LOM). As a result, the d-NiOOH displays excellent performance for the OER with an overpotential of 201 mV at 100 mA cm−2, surpassing the ordered NiOOH (o-NiOOH, 286 mV). Remarkably, an anion exchange membrane water electrolyzer (AEMWE) assembled with a-NiMoO as the anodic catalyst can attain a large current density of 1 A cm−2 at a small voltage of 1.79 V, outperforming most of the reported electrocatalysts.
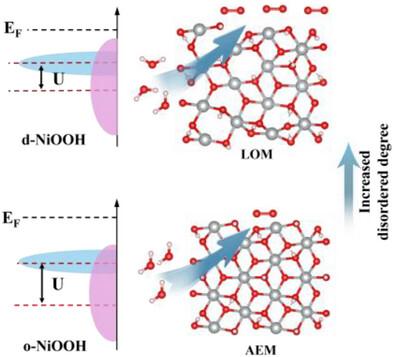
电化学重构诱导无序NiOOH生成,改变析氧反应机理
析氧反应中电催化剂的电化学重构行为是决定其性能的关键。尽管它的关键作用,精确控制和合理指导这种重建行为仍然是一个难以捉摸的挑战。本文报道了一种有效的策略,通过同时引入非晶结构和易氧化元素(即Mo6+)来操纵镍氧化物的重建行为。具体来说,非晶结构促进了低电位重构和Mo6+的氧化去除,从而生成了具有丰富缺陷的无序NiOOH (d-NiOOH)。值得注意的是,d-NiOOH显著增强了Ni-O共价,从而触发了反应机制从吸附演化机制(AEM)向晶格氧介导机制(LOM)的转变。结果表明,d-NiOOH在100 mA cm−2下的过电位为201 mV,优于有序NiOOH (o-NiOOH, 286 mV)。值得注意的是,以a- nimoo为阳极催化剂组装的阴离子交换膜水电解槽(AEMWE)在1.79 V的小电压下可以获得1 a cm−2的大电流密度,优于大多数电催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


